High Solubility and Partial Molar Volume of Hexyl Methyl Malonate in Supercritical Carbon Dioxide
Yang Haijian, Zhao Lu, Xiang Li, Cai Zhuofu
(College of Chemistry and Materials Science, South-Central University for Nationalities, Wuhan 430074, China )
Supercritical carbon dioxide (scCO2) has been receiving increasing attentions for many applications as reactant in the field of chemistry and chemical industry due to its unique properties such as liquid-like density and solvent power, gas-like transport properties, sensitivity of the properties with critical constants, and its benignity to environment[1-3].
However, the non-polarity of CO2limits the further utilizations by the need for high CO2pressure to dissolve even small amounts of polar, silicone-functional amphiphilic, organometallic, or high-molecular-mass compounds[4,5]. And compounds which have a perfluoroalkyl polyether (PFPE) tails showing high solubilities in supercritical CO2, but this type of compounds are very expensive and toxic[6].
Generally, the solubility of compounds depends on their groups of molecular structure, especially the end groups and molecular weight. So the design and synthesis of highly CO2-soluble compounds via end group modification would greatly benefit the potential applications of CO2as a solvent. According to the literature and based on our research results, hydrocarbons substituted with carbonyl groups as CO2-philes have appeared economically, and the carbonyl group, ether group, and alkyl group with suitable length are so-called CO2-philic groups[7-9]. So,we designed and synthesized a new compound——hexyl methyl malonate via end group modification, which has carbonyl group, and alkyl group with suitable length. The solubilities of compound in scCO2over the pressure ranging from 8.5 MPa to 13.6 MPa and at temperatures 313 K, 333 K and 353 K were determined. The tested results were also correlated by Bartle and Chrastil models. And the partial molar volume was calculated according to Kumar-Johnston theory.
1 Experimental
1.1 Reagents and Apparatus
Methyl malonyl chloride (w=0.97, mass fraction, CAS Registry No.37517-81-0), 1-hexanol(w=0.99, mass fraction, CAS Registry No.111-27-3, Alfa Aesar Chem. Co.) Triethylamine (w=0.99, mass fraction, CAS Registry No.121-44-8, Acros Organics Chem. Co.). CO2(w=0.9999, mass fraction, Wuhan Steel Co.). Dichloromethane (Tianjin Kemel Co. Ltd.). CO2delivery pump(JASCO PU-CO2), back pressure regulator(JASCO BP-1580-81), NMR experiments(JEOL Al-600MHz).
1.2 Synthesis of hexyl methyl malonate
The target compound hexyl methyl malonate was synthesized according to the method shown as Fig.1[10].

Fig.1 Synthesis of hexyl methyl malonate圖1 甲氧羰乙酸己酯的合成
1-Hexanol (2.6 mL, 20.702 mmol) was added into a 100 mL flask under N2atmosphere, then a CH2Cl2solution (30 mL) of methyl malonyl chloride (1 mL, 9.407 mmol) and triethylamine (2.8 mL, 20.17 mmol) was added dropwise to the flask. The mixture was stirred at room temperature for a whole night, then the reaction mixture was washed twice with aq. HCl (0.05 mol/L), saturated aq. NaHCO3, and deionized water. The organic phase was collected and dried over anhydrous Na2SO4. After filtration and evaporation under vacuum, the residue was purified by silica gel column chromatography with ethyl acetate-petroleum ether( volume proportion is 1 to 5) as eluent to give a yellow oil (GC purity>99%) with 83 % yield.1H NMR (CDCl3)δ: 4.131~4.120 (m,J=4.4, 2H, CH2O), 3.730 (s, 3H, OCH3), 3.365 (s, 2H, COCH2CO), 1.789~1.290 (m,J=64.8, 8H, CH2), 1.029~0.722 (t, 3H, CH3).13C NMR (CDCl3)δ:166.71,70.06,65.65,52.36,41.34,31.30,28.36,25.38,22.45,13.89. Elemental Anal: C10H18O4found C 59.41%, H 8.91%, O 31.68 %; required C 59.43%, H 8.89 %, O 31.68 %.
2 Results and Discussion
2.1 Solubility results
The solubility data of Hexyl methyl malonate tested at different conditions of pressures (8.5 MPa to 13.6 MPa) and temperatures (313 K,333 K and 353 K) in scCO2were listed in Tab.1. As shown in Tab.1, the solubility of compound increased with the increase of pressure at the same temperature, while at the same pressure, the solubility decreased with the increase of temperature, mainly because the solvent power of CO2varied with the change of CO2density at different pressures or temperatures.
2.1.1 Bartle model
The experimental solubility data for the compound were correlated using the following equation.
ln(xP/Pref)=A+C(ρ-ρref).
(1)
where
A=a+b/T.
(2)
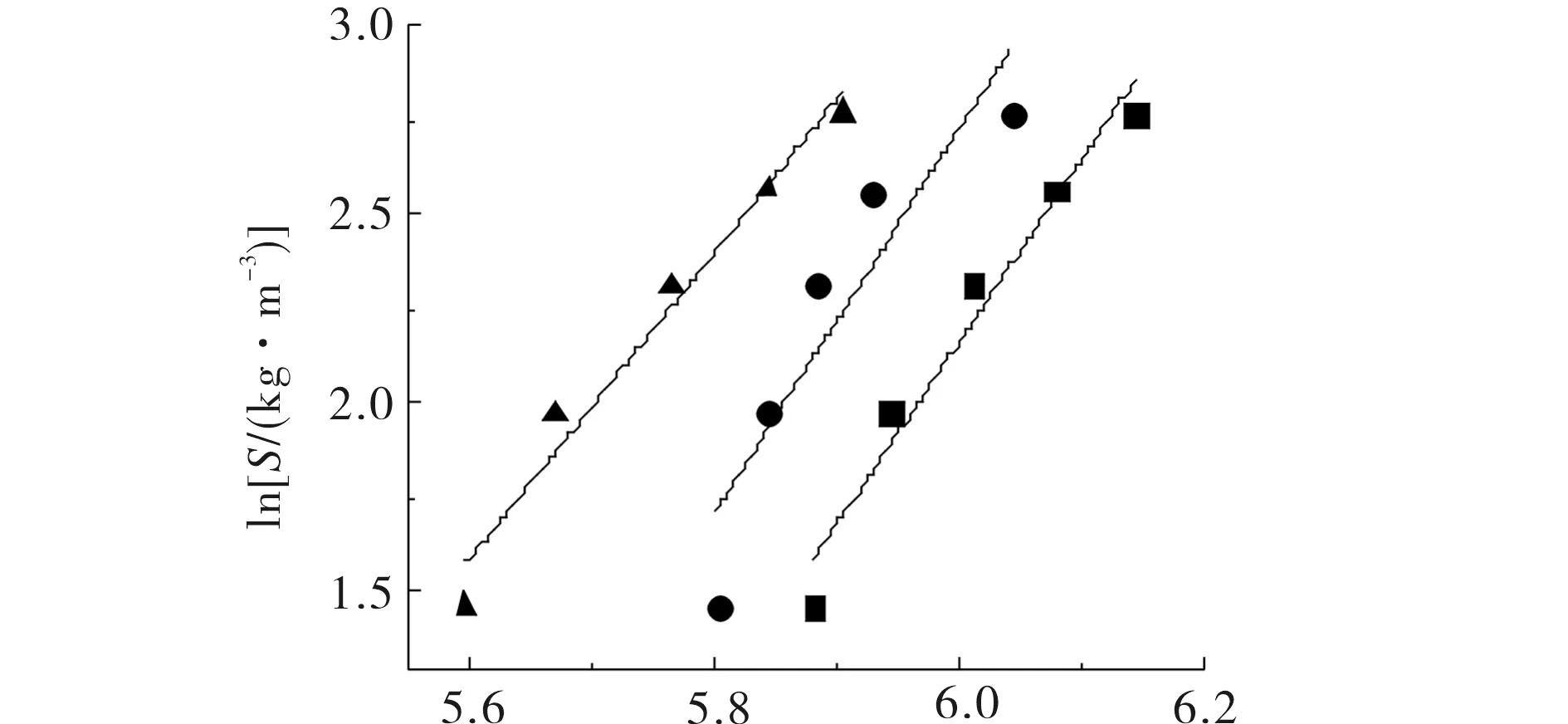
wherexis the mole fraction of the solutes;Pis the pressure;Prefis 0.1 MPa;ρis the density of pure CO2at the experimental temperature and pressure;ρrefis 700 kg·m-3; and A, C, a and b are constants. At the initial stage, ln (xP/Pref) values were plotted against (ρ-ρref) (Fig.2), and the values were fitted with a straight line by least-squares regression to estimate theCandAparameters. TheCvalues, obtained from the slopes of the corresponding plots, were averaged for each compound (Tab.2). WhenCwas held at its average value, the experimental solubility data were used to evaluateAvalues at various temperatures. The plots ofAversus 1 /Twere fitted to a straight line (Fig.2) from which the intercept and the slope (aandb) were obtained. The resulting a and b values were shown in Tab.2. Thea,b, andCvalues were used to predict solubility using Eq (1) and Eq (2). In this model, the parameterbis related to the enthalpy of sublimation of the solute,ΔsubH, by the expressionΔsubH=-Rb, whereRis the gas constant[11].

Tab.1 Solubility of C10H18O4 at different temperatures, densities, and mole fractions in supercritical carbon dioxide.

Tab.2 Results of the solubility data correlation by Bartle and Chrastil model
-:undone data

(ρ-ρref)/(kg·m-3) ■313 K;●333 K;▲353 KFig.2 Plots of ln (xP/Pref) vs (ρ-ρref) for hexyl methyl malonate at various temperatures by Bartle model 圖2 甲氧羰基乙酸己酯由Bartle 模型模擬在不同溫度下關聯(lián)的ln(xP/Pref) vs (ρ-ρref) 擬合數(shù)據(jù)
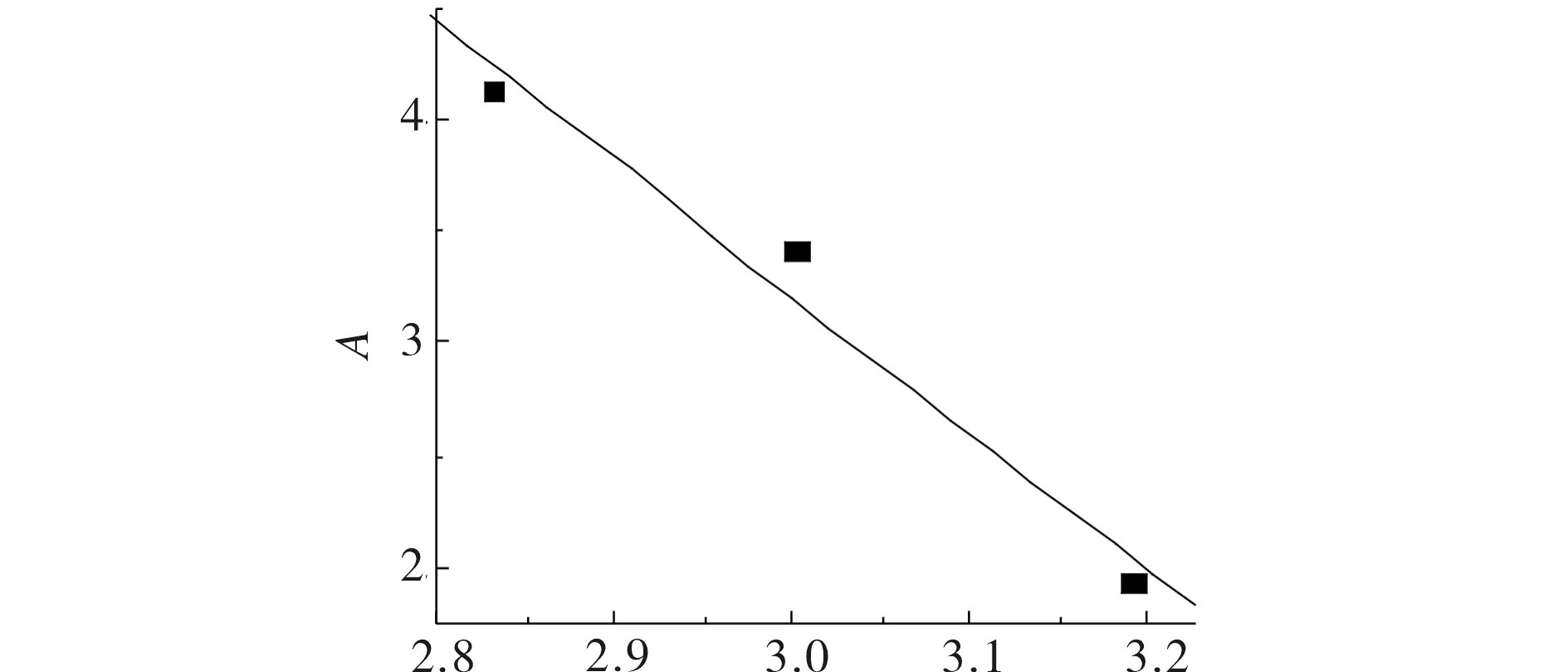
T/103KFig.3 Plots of A vs 1/T for hexyl methyl malonate圖3 甲氧羰基乙酸己酯關聯(lián)的A對1/T擬合數(shù)據(jù)
The average absolute relative deviation (AARD) was used to test the correlation results. It was calculated with the following Eq (3).
AARD=1/nS|(xcal-xexp)/xexp|×100 %.
(3)
wherenwas the number of experimental points, andxcalandxexpwere the calculated and experimental data respectively. The values ofAARDwere in the range of 3.28 %~58.65 %.
2.1.2 Chrastil model
In this model, the experimental solubility data for the compound were correlated by Eq (4).
lnS=klnρ+(α/T)+β.
(4)
where the solubility(S) was calculated by Eq (5).
S= (ρM2x) /[M1(1-x)].
(5)
wherexis the molar fraction of the solute,M1andM2are the molecular weights of CO2and the solute. The constantsα,βandkcan be estimated from the experimental solubility data in scCO2.ρis the density of the pure scCO2;Sthe solubility of the solid in the supercritical phase;Tthe temperature inK;k,αandβare the adjustable parameters of the model. The constantkis the association number,αa constant, defined as -ΔH/R(whereΔHis the sum of the enthalpies of vaporization and solvation of the solute andRthe gas constant) andβdepends on the molecular weights of the solute and solvent. The Chrastil model suggests that plots of lnSfor several temperatures are straight lines whose slopes are identical and equal tok. The parameters,k,αandβare obtained performing a multiple linear regression on the experimental solubility data[12,13].
The values of calculated constants for the Hexyl methyl malonate in scCO2systems were presented in Tab. 2. The quality of the correlation is expressed in terms ofσ2andAARDbetween experimental and calculated solubilityS. The consistency of the model with measured data can be seen from Fig.4 and the values ofAARDat different temperatures, which are between 0.0068 % ~27.7 %. The results exhibited the good agreement between the tested and calculated data.
ln[ρ/(kg·m-3)]■ 313 K;● 333 K ;▲353 K Fig.4 Plots of lnS vs lnρ for hexyl methyl malonate at different temperatures by Chrastil model圖4 甲氧羰基乙酸己酯由Chrastil 模型擬在不同溫度下關聯(lián)的lnS對lnρ擬合數(shù)據(jù)
2.1.3 Estimation of the partial molar volumes of the solutes
According to Kumar and Johnston[14], the dependence of the solubilityxof the solute with its partial molar volume in the vicinity of the critical density of the SCF, can be expressed by the following equation.

(6)


(7)
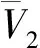
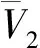

Tab.3 Slopes and the corresponding partial molar volumes of the solute for hexyl methyl malonate in scCO2 system at different temperatures 表3 不同溫度下甲氧羰基乙酸己酯在超臨界體系中的偏摩爾體積和相關參數(shù)

lnρ ■ 313 K;● 333 K ;▲ 353 KFig.5 Plots of ln x vs lnρr for hexyl methyl malonate at different temperatures圖5 甲氧羰基乙酸乙酸在不同溫度下關聯(lián)的lnx對lnρr擬合數(shù)據(jù)
3 Conclusion

Acknowledgements
We are grateful to National Natural Science Fundation of China (No. 2067031), the finacial support of Beijing National Laboratory for Molecular Sciences (BNLMS) and the valuable help of Prof. Buxing Han.
[1] Yang H J, Kim H, Guo C Y. Metal ion extraction with bipyridine derivatives as chelating ligands in supercritical carbon dioxide [J].Clean-Soil Air Water, 2010, 38(2): 159-166.
[2] 楊海健, 金 晶, 張 寧, 等. 二甘醇單醚類化合物在超臨界二氧化碳中的溶解行為[J].中南民族大學學報: 自然科學版, 2011, 30(4): 1-5.
[3] Yang H J, Sun W H, Li Z L. Solvent-free syntheses of salicylaldimines assisted by microwave irradiation [J]. Syn Commun, 2002, 32(15): 2395-2402.
[4] Yang H J,Sun W H,Chang F,et al. Vinyl polymerization of norbornene catalyzed by salicylaldiminato nickel(II) complexes/MAO system [J]. Appl Catal A-General, 2003, 252(2): 261-267.
[5] Yang G C, Yang H J. Metal ions extraction with glucose derivatives as chelating regents in supercritical carbon dioxide [J]. Chin Chem Lett, 2006, 17(11): 1489-1492.
[6] 楊海健, 張 寧, 金 晶, 等. 乙二醇單醚在超臨界二氧化碳中溶解度的研究及關聯(lián)[J].中南民族大學學報:自然科學版, 2011, 30(3): 1-6.
[7] Bai Y, Yang H J, Quan C, et al. Solubilities of 2,2′-bipyridine and 4,4′-dimethyl-2,2′-bipyridine in supercritical carbon dioxide [J]. J Chem Eng Data, 2007, 52(5): 2074-2076.
[8] Yang H J. Nonfluorous, highly CO2-soluble chelating ligands for scCO2metal ions extraction [J]. Chem Lett, 2006, 35(9): 1000-1001.
[9] Bie P, Yang H J, Bai Y. New CO2-philic compounds: design, synthesis and solubility in supercritical carbon dioxide [J]. Chin Chem Lett, 2006, 17(6): 743-746.
[10] Kawakami T, Saito N, Ikushima Y, et al. Highly-soluble fluorocarbon surfactant in supercritical carbon dioxide: effect of counter cation on solubility [J]. Chem Lett, 2000, 29(4): 402-403.
[11] Tian J, Yang H J, Wang W, et al. Metal ions extraction from solid matrix in supercritical carbon dioxide with DMBP as chelating ligand [J]. Clean-Soil Air Water, 2010, 38(5): 543-547.
[12] Miller D J, Hawthrone S B, Clifford A A, et al. Solubility of polycyclic aromatic hydrocarbons in supercritical carbon dioxide from 313 K to 523 K and from 100 bar to 450 bar [J]. J Chem Eng Data, 1996, 41(4): 779-786.
[13] Bartle K D, Clifford A A, Jafar S A, et al. Solubilities of solids and liquids of low volatility in supercritical carbon dioxide [J]. J Phys Chem Ref Data, 1991, 20(4): 713-756.
[14] Wang W, Yang H J, Hu J C, et al. Solubilities of diglycolic acid esters at temperatures ranging from (343 to 363) K in supercritical carbon dioxide [J]. J Chem Eng Data, 2010, 55(2): 694-697.
[15] Dandge D K,Heller J P,Kennard V. Structure solubility correlations organic compounds and dense carbon dioxide binary systems [J]. Ind Eng Chem Prod Res Dev,1985,24(1): 162-166.

