A Phosphoric Acid Pretreatment of Activated Carbon Supported Pd Catalyst for Acetylene Hydrochlorination?
WANG Feng,MA Lei,WANG Ji-de
(Key Laboratory of Oil and Gas Fine Chemicals,Ministry of Education and Xinjiang Uyghur Autonomous Region,Xinjiang University,Urumqi,Xinjiang,830046,China)
Abstract: Activatedcarbon(AC)pretreated with phosphoricacid(H3PO4)supported Pdcatalyst(PACT)wasprepared usingthe impregnationmethod.Catalytic activityofthe catalyst wasevaluatedinthe acetylenehydrochlorinationreaction.The results show that the catalyst yield superior catalytic performance compared to the catalytic(PAC),in which the activated carbon support was untreated.The characterization of surface area(BET),Boehm titration measurement,X-ray diffraction(XRD),transmission electron microscopy(TEM),and energy-dispersive spectrometry(EDS)demonstrated that concentration of acidic oxygen-containing functional groups on the surface of the PACT,Pd-containing active species and introducingby H3PO4were responsible for the high activity of the optimal Pd catalyst.Thermo Gravimetric analysis(TG)indicated that carbon deposition produced by the reaction lead to the decrease of activity.
Key words:Activated carbon;H3PO4;Palladium catalyst;Acetylene hydrochlorination
0 Introductions
Acetylene hydrochlorination is a very important industrial process for the production of vinyl chloride monomer(VCM),which is further used as the starting material for polyvinyl chloride production[1].
Currently,the reaction is primarily performed under the catalysis of HgC12supported on activated carbon.But during the reaction the highly toxic HgC12tends to sublime,resulting in severe environmental pollution problems.Hence,development of nonmercuric catalyst,therefore,has been a challengeable task in the acetylene hydrochlorination.Activated carbon supported noble metal catalysts were reported by some groups.Hutchings researched the correlation between the activities of some metal chloride catalysts and the standard electrode potential as well as predicted that gold would be the best catalyst for hydrochlorination of acetylene[2-4].Conte et al.investigated the effect of adding Pd,Ir,Rh,Pt,or Ru on the activity of Au-based catalyst.Pd and Pt modi fied Au catalysts both gave a higher initial activity but deactivated rapidly due to serious coking.Ir and Rh modi fied ones exhibited enhanced activities with little change in selectivity and Ru modi fied catalyst had no signi ficant effect on catalytic activity of the Au catalyst.They also carried out a series of tests in which the gold catalyst was sequentially exposed to respective reactant(C2H2,HCl)for a period of time.They founded that exposure to HCl leads to enhanced activity but exposure to C2H2leads to deactivation[5-7].Wang et al.concluded that the gold catalyst on active carbon with a proper micropore size exhibited a higher activity than a smaller micropore size[8].Wang et al.reported that the catalytic activity of PdCl2supported on active carbon catalyst was increased by addition of KCl and LaCl3[9].Yang et al.developed a PdCl2,PtCl4catalyst which have the performance of about 91%~96%conversion of acetylene and about 99%selectivity for VCM[10].The studies above focus on the activate component,few on the support.Some researches point out that support pretreatment is necessary for some reactions[11-14].Effect of pretreatment is not only on the physical structure but also on surface functional ions or radicals(mainly carboxylic,laconic and hydroxyl groups)of the support.As a result the catalytic properties were enhanced.
In this work,Pd catalyst supported activated carbon,which was pretreated with phosphoric acid,was prepared and invested in acetylene hydrochlorination.The result showed that the catalyst exhibited higher catalytic activity then the catalyst which support was untreated.The catalyst was characterized by BET,Boehm titration measurement,XRD,TEM,and EDS-SEM,and the reason about high catalytic activity and deactivation of the catalyst were discussed.
1 Experimental
1.1 Catalyst preparation
The AC material was firstly pretreated with different concentrations of H3PO4(5wt%,10%,15%,20%,and 25%)solutions at 80?C for about 6 h under reflux.Then the samples were washed with distilled water and dried in air at 105~110?C for 3 h.The pretreated-supporters were denoted as AC1,AC2,AC3,AC4,and AC5,respectively.Pdbased catalyst(PACT)were prepared by impregnation method:the pretreated AC supports were impregnated with an acidic PdCl2solution in an appropriate concentration to obtain Pd loadings of about 0.5%under stirring,then use the ultrasonic to dispersion,dried at 120?C for 4 h, finally.This series of catalysts were marked as PACT1,PACT2,PACT3,PACT4,and PACT5,respectively.
1.2 Catalyst characteristics
The surface areas of the samples were measured on JW-BK Brunauer-Emmett-Teller(BET)equipment.The amounts of Oxygen-containing functional groups on the AC surface were determined by Boehm titration measurement.X-ray diffraction(XRD)data were carried out on a Rigaku D/max-ga X-ray diffractometer with Cu-Kα radiation.The transmission electronic microscopy(TEM)analysis was conducted on a model JEOL JEM-2100 with an accelerating voltage of 200 kV.The composition of catalyst was estimated by an energy-dispersive spectrometry(EDS)analyzer attached to a LEO1450VP scanning electron microscope(SEM).Thermo Gravimetric(TG)curve was detected by a NETZS STA449C differential thermal analyzer.
1.3 Catalyst Testing
Catalytic performance of the catalysts for acetylene hydrochlorination was tested in a fixed-bed reactor operating just above atmospheric pressure.After puri fied and dried,hydrogen chloride and acetylene,at the ratio of 1.15,were regulated fed into the reactor using mass flowmeters.Catalyst dosage was 4~5 g,total GHSV was 120 h?1and the reaction temperature was 160?C.The effluent from the reactor was passed through a vessel filled with sodium hydroxide solution to remove the unreacted hydrogen chloride,and then was analyzed by gas chromatography.
2 Results and discussion
2.1 Catalytic Performance
The effect of different concentrations of H3PO4(from 5 to 25%)on the performance(acetylene conversion and selectivity of vinyl chloride)of the PACT catalysts is shown in Figure 1.It is obviously that acetylene conversions(about 95~97%)of PACT catalysts are higher than that(about 80%)of the untreated one.For the PACT catalysts the acetylene conversion increases gradually with increasing H3PO4concentration then decrease slightly.The catalysts showed high selectivity to vinyl chloride(more than 99%)whether they were pretreated or not,indicating the fewer by-products was formed in acetylene hydrochlorination reaction.From the results above,it is regarded that pretreated with H3PO4can improve the catalytic activity of the catalyst,while the in fluence of the H3PO4concentration is not appreciable.
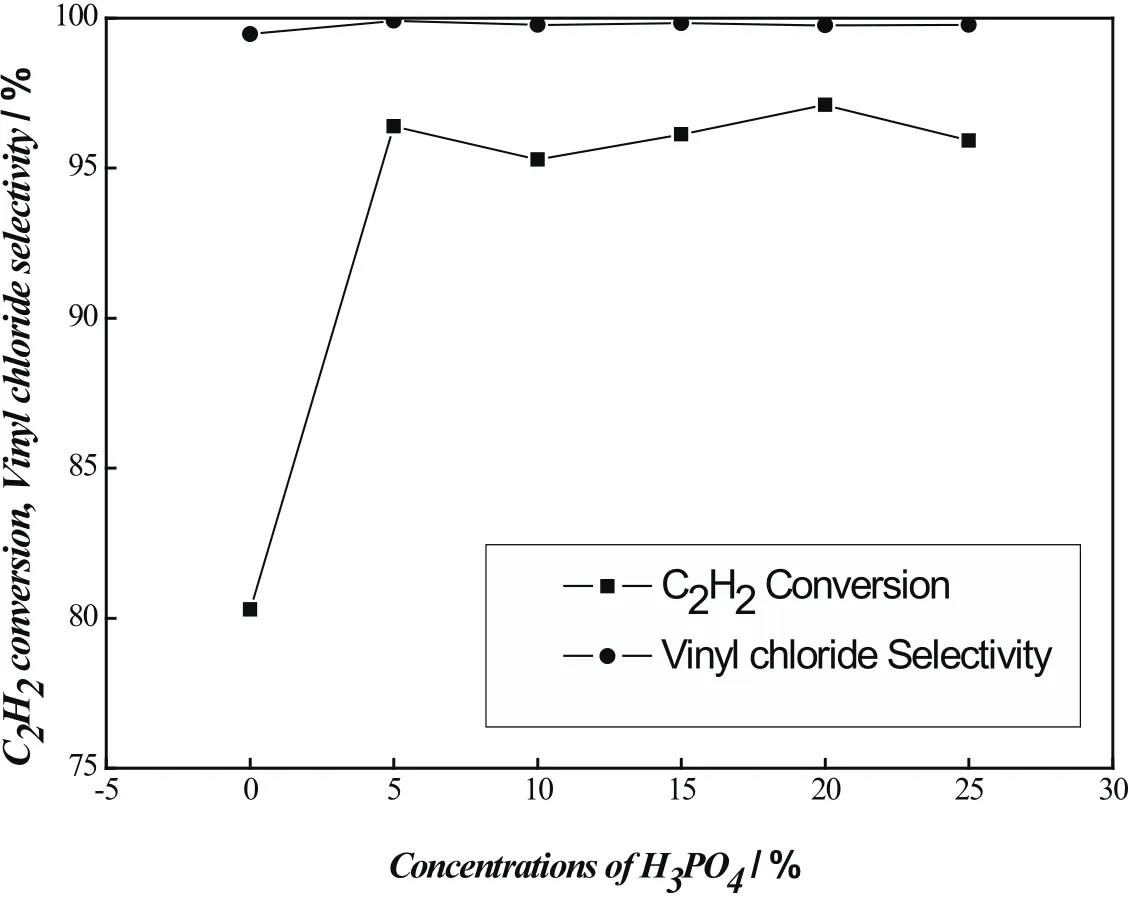
Fig 1 catalytic performance of Pd the catalysts
2.2 Catalyst characteristics
For studying the in fluence of pretreatment,the physical and chemical properties of the support were detected.Some parameters are summarized in table 1 and 2.Table 1 show the specific surface areas and pore structure of AC without pretreated and pretreated,table 2 shows the surface acidity by Boehm’s method.Comparison with the parent AC,the specific surface area,average pore volume,diameter and surface acidic oxygen-containing groups of the acidtreated AC were increased.It most due to H3PO4which cleaned up residue of the pores in the support AC[14]and react with some surface functional groups on the support AC.The oxygen-containing functional groups on the support can act as anchoring sites which are depositional center of metal component[15],make precursor solution closer the surface of AC and improve the catalytic activity obviously[14].Moreover,the H3PO4can provide proton,enhance the formation of π-complex consisted of acetylene and Pd(Ⅱ).Consequently,the activity of the catalyst is improved.
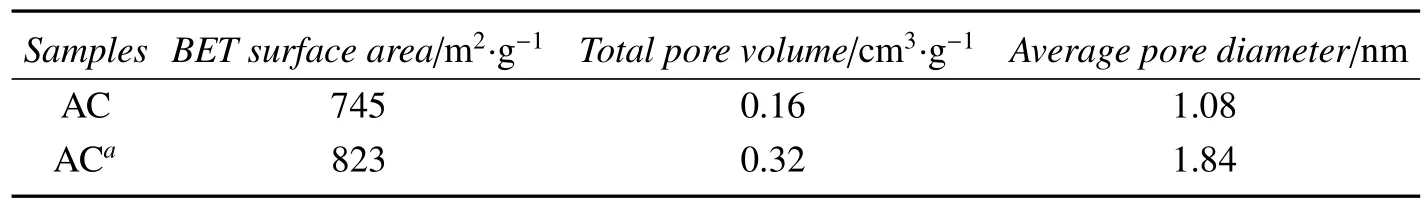
Table 1 Porous structure parameters of the supporters
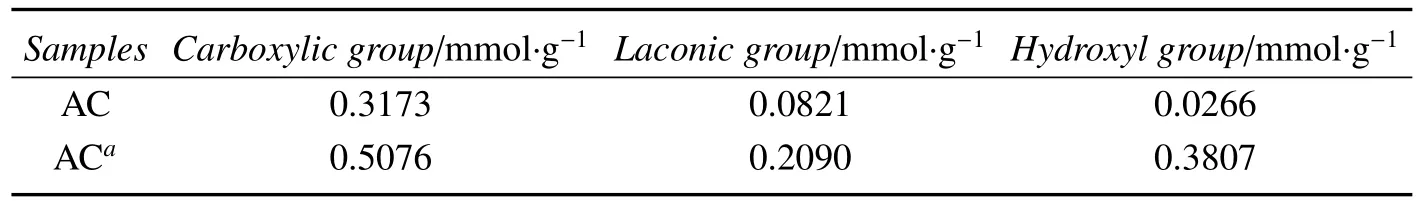
Table 2 Surface acidity of AC by Boehm’s method
XRD characterization of the pretreated support and PACT catalysts with different Pd loading in Fig.2 shows that the XRD patterns of catalyst are very similar to that of the support although the Pd loading was increased 5wt%and no any peaks of Pd species were detected.This suggests that active component Pd is high dispersive[16].
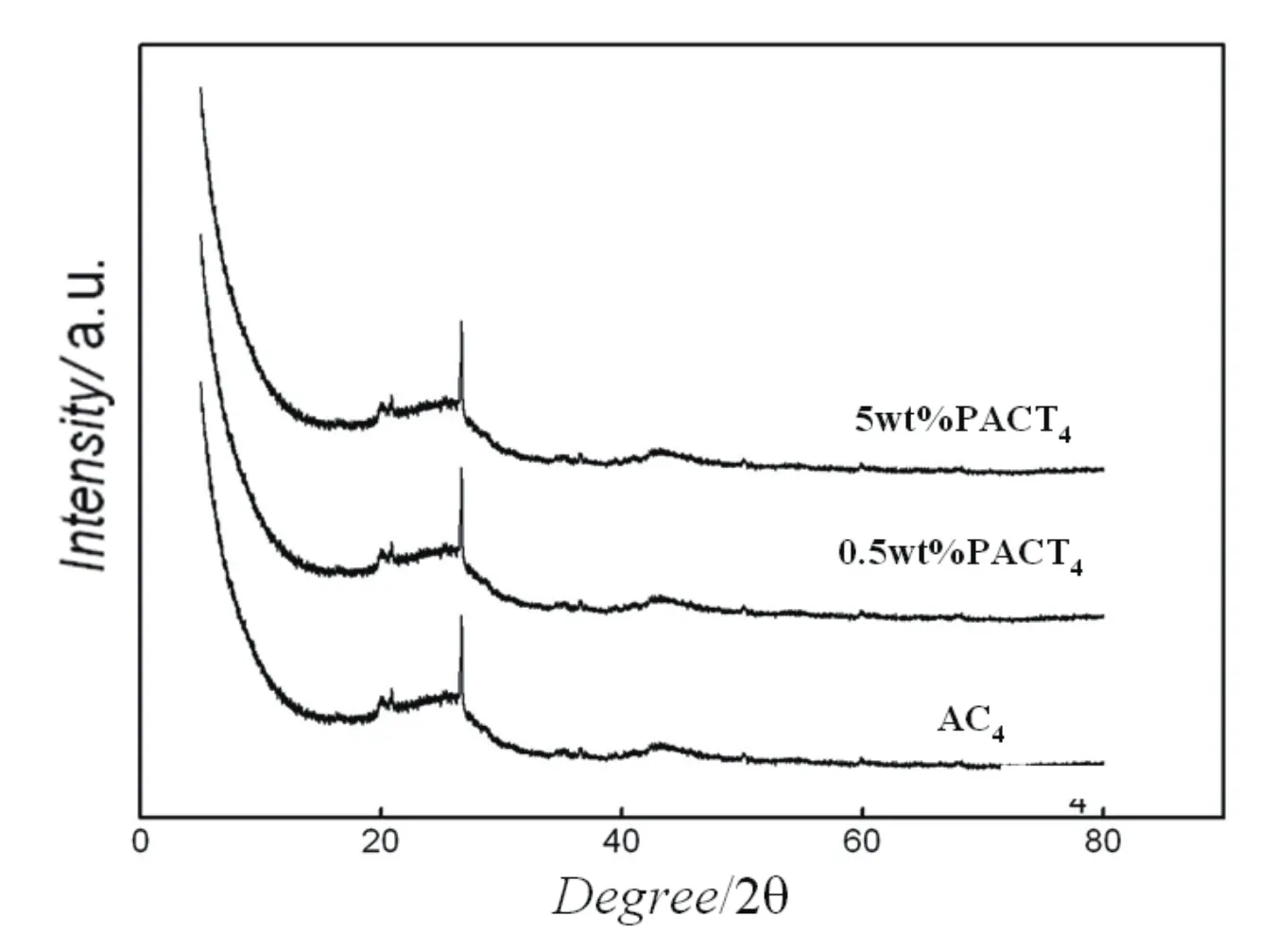
Fig 2 The XRD patterns of the samples
From TEM images of AC untreated and treated supported Pd catalysts.It can be seen that Pd active species are relatively uniformly dispersed on the surface of the both supports.This result is in good agreement with the observations from XRD analysis.It also can be observed that the acid-treated support catalyst possess more remarkable particle and dense Pd active species than that of untreated supported one.
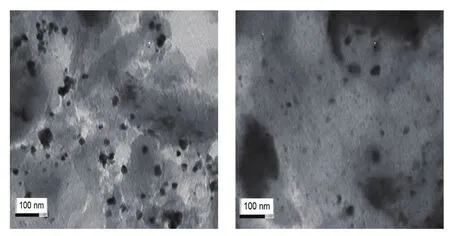
Fig 3 a TEM image of the PACT,b TEM image of the PAC
For detecting the chemical composition and content of each element in the samples EDS characterization was carry out.The results in Fig.3 show that Pd content on the pretreated AC is 8.18%higher than that of on the untreated AC one(1.48%).This indicated that perpetration of H3PO4make Pd content rich on the surface of the support.This is agreement with the result of TEM.
2.3 Discussion of the catalyst deactivation
It was found that the pretreated support catalyst was deactivated after about 3 h.In order to explain the catalyst deactivation,TG and analysis were conducted.TG curves of fresh and deactivated were shown in Fig.5.The fresh catalyst had a loss of mass before 100?C.This is due to desorption of the adsorbed water on the surface of the catalyst.When temperature exceeded 700?C,there was a gradually weightlessness which is caused by burning carbon.While the deactivated catalyst had an obvious weightlessness between 100?C and 700?C.Wang regard as carbon deposition from the reaction is the reason of the weightlessness[19].Porous structure parameters listed in Table 3 show that the surface area and pore volume deactivated catalyst are signi ficantly reduced but the average pore diameter is increased.This can prove that narrow pores of the catalyst are blocked carbon deposition which covers the active sites on the surface of catalyst and leads to the catalyst deactivation[17].
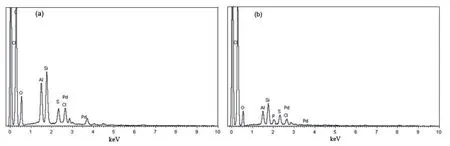
Fig 4 a EDS spectrum of PAC,b EDS spectrum PACT catalyst

Fig 5 TG curves of fresh and deactivated PACT catalysts
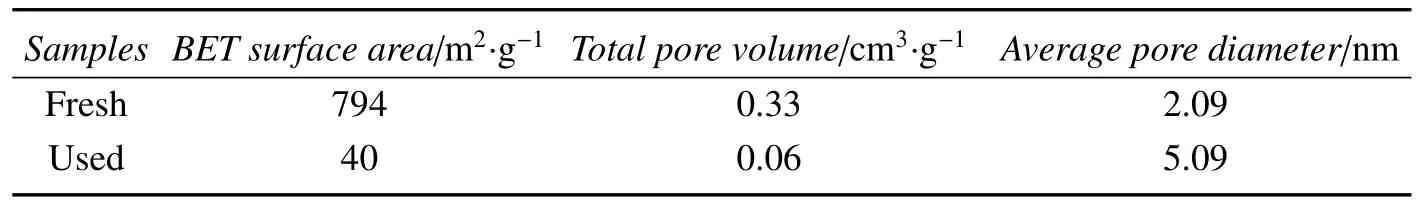
Table 3 Porous structure parameters of PACT catalysts
3 Conclusions
Pretreatment AC support with H3PO4can improve the activity of supported Pd catalyst for the Acetylene hydrochlorination.Because of the increase of acidic oxygen-containing functional groups and Pd-containing active species on the surface of the catalyst,and H3PO4which provide proton,enhance the formation of π-complex consisted of acetylene and Pd the catalyst perform higher activity then that of the catalyst without pretreatment.Carbon deposition is primary reason for the deactivation of the catalyst.

