遠程缺血預處理對大鼠體外循環后肺損傷的保護作用及機制探討
涂 杰,梁東科,劉國鋒,梁蓓薇,韋秋英,劉菊梅,張炳東
(廣西醫科大學第一附屬醫院心血管病研究所手術麻醉室,廣西 南寧 530021)
遠程缺血預處理對大鼠體外循環后肺損傷的保護作用及機制探討
涂 杰,梁東科,劉國鋒,梁蓓薇,韋秋英,劉菊梅,張炳東
(廣西醫科大學第一附屬醫院心血管病研究所手術麻醉室,廣西 南寧 530021)
目的 評價遠程缺血預處理(RIPC)對大鼠體外循環(CPB)后肺損傷的保護作用并探討相關機制。方法成年雄性SD大鼠60只,隨機分為三組(n=20):假手術組(S組)、CPB組和RIPC+CPB組。S組行麻醉和穿刺但不行CPB;CPB組采用尾動脈插管灌注,右頸靜脈插管引流建立CPB模型;RIPC+CPB組在實施CPB前對后肢進行RIPC(通過驅血帶阻斷后肢血流10 min,復灌10 min,兩側后肢交替進行,每側進行3次)。麻醉復蘇后2 h采集上腔靜脈血樣,測定血清TNF-α和IL-6濃度,然后處死大鼠取肺組織,測定肺濕/干重比值(W/D比值)、丙二醛(MDA)含量和谷胱甘肽過氧化物酶(GSH-px)活性,光鏡下觀察病理學改變。結果與S組比較,CPB組和RIPC+CPB組血清TNF-α和IL-6濃度升高,肺組織W/D比值和MDA含量升高,GSH-px活性降低(P<0.05);與CPB組比較,RIPC+CPB組血清TNF-α和IL-6濃度下降,肺組織W/D比值和MDA含量下降,GSH-px活性升高(P<0.05)。HE染色顯示RIPC+CPB組肺組織的形態學損傷輕于CPB組。結論RIPC可減輕大鼠CPB后肺損傷,其機制與抑制炎性反應和脂質過氧化反應有關。
遠程缺血預處理;體外循環;肺損傷
體外循環(Cardiopulmonary bypass,CPB)誘發的腸道內毒素轉移、缺血再灌注損傷和全身炎性反應等均可引起不同程度的肺損傷,輕者僅表現為亞臨床癥狀的功能改變,重者則表現為急性呼吸窘迫綜合征,嚴重影響術后肺功能的恢復[1]。遠端缺血預處理(Remote ischemic preconditioning,RIPC)指通過對一個組織或器官進行短暫的非致死性缺血再灌注后,可加強遠隔臟器對隨后較長時間缺血的耐受能力。RIPC實施方便,對機體幾乎無損傷,可明顯減輕遠隔臟器缺血再灌注損傷,但具體機制尚不清楚[2]。因此,本研究擬采用大鼠CPB模型,觀察RIPC對大鼠CPB后肺損傷的影響,并探討相關機制,為臨床應用提供依據。
1 材料與方法
1.1 主要儀器與試劑 S5型多功能監測儀(美國Datex-ohmeda公司),StockertⅢ型雙頭滾壓泵(德國Stockert公司),小動物膜式氧合器(東莞科威醫療器械有限公司),腫瘤壞死因子-α(TNF-α)和白細胞介素-6(IL-6)試劑盒(北京科美東雅生物技術有限公司),丙二醛(MDA)和谷胱甘肽過氧化物酶(GSH-px)試劑盒(南京建成生物工程研究所)。
1.2 動物選擇及分組 本實驗經廣西醫科大學動物委員會批準,并符合國家科技部《關于善待實驗動物的指導性意見》的規定。成年雄性SD大鼠60只,清潔級,6~8個月齡,體重400~450 g,由廣西醫科大學實驗動物中心提供。采用隨機數字表法分為三組:假手術組(S組)、CPB組和RIPC+CPB組,每組20只。S組僅于麻醉誘導后行機械通氣和各部位插管,不行CPB;CPB組建立CPB模型;RIPC+CPB組實施CPB前,對后肢行RIPC。
1.3 RIPC方法 通過驅血帶阻斷后肢血流10 min,復灌10 min,兩側后肢交替進行,每側進行3次,阻斷壓力以多普勒血流探測儀監測后肢動脈搏動正好消失為宜,方法同文獻[3]。
1.4 CPB模型制備 參照文獻[4]制作大鼠CPB模型。腹腔注射10%烏拉坦10 ml/kg麻醉,16G靜脈導管行氣管插管后機械通氣。采用尾動脈插管灌注,右頸靜脈插管引流建立循環通路。無血預充,灌注流量約為100 ml/kg,氧流量與灌注流量之比為0.8,通過變溫裝置保持肛溫37℃,α穩態管理血氣,術中加入新鮮大鼠肝素血,維持血細胞比容在0.20~0.25。CPB轉流2 h后停機,管道剩余血液經離心濃縮后緩慢回輸。待自主呼吸恢復平穩后停止機械通氣,拔除氣管導管,并密切觀察大鼠麻醉復蘇后生命體征。
1.5 標本采集及檢測
1.5.1 血清TNF-α和IL-6濃度的檢測 麻醉復蘇后2 h,采集上腔靜脈血5 ml,4℃下3 000 r/min離心5 min后取上清液,按試劑盒說明書以雙抗體夾心酶聯免疫吸附法檢測TNF-α和IL-6濃度。
1.5.2 肺濕/干重比值(W/D比值)測定 大鼠上腔靜脈血標本采集完畢后,剖胸取左肺下葉,濾紙吸去表面血跡后稱濕質量,然后置于70℃烤箱內烘干至恒重,稱干質量,計算W/D比值。
1.5.3 肺組織MDA含量和GSH-px活性測定取右肺下葉,濾紙吸干表面污物,制備10%組織勻漿,考馬斯亮藍法測定勻漿內蛋白濃度,然后按試劑盒說明書測定MDA含量和GSH-px活性。
1.5.4 肺組織病理學情況觀察 取左肺上葉組織,4%多聚甲醛固定,石蠟包埋切片,HE染色,光鏡下觀察病理學情況。
1.6 統計學方法 采用SPSS15.0統計學軟件進行分析,計量資料以均數±標準差(±s)表示,多組間比較采用單因素方差分析,以P<0.05為差異有統計學意義。
2 結果
2.1 肺組織病理學觀察結果 S組肺組織結構完整,無明顯充血、水腫及炎性細胞浸潤;CPB組肺間質水腫、出血,肺泡間隔增寬,肺泡腔內可見大量炎性細胞浸潤;RIPC+CPB組肺間質略水腫,少量出血,肺泡間隔輕度增寬,肺泡腔內少量炎性細胞浸潤,見圖1。
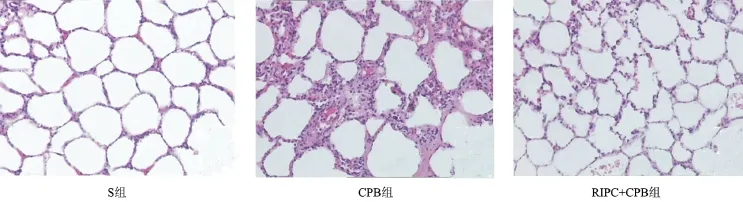
圖1 光鏡下三組肺組織病理學檢查結果(HE染色,×100)
2.2 血清TNF-α和IL-6濃度比較 與S組比較,CPB組和RIPC+CPB組血清TNF-α和IL-6濃度升高(P<0.05);與CPB組比較,RIPC+CPB組血清TNF-α和IL-6濃度下降(P<0.05),見表1。
2.3 肺組織W/D比值、MDA含量和GSH-px活性比較 與S組比較,CPB組和RIPC+CPB組肺組織W/D比值和MDA含量升高,GSH-px活性降低(P<0.05);與CPB組比較,RIPC+CPB組肺組織W/D比值和MDA含量下降,GSH-px活性升高(P<0.05),見表1。
表1 三組大鼠血清TNF-α、IL-6、肺組織W/D比值、MDA和GSH-px活性比較(n=20,±s)
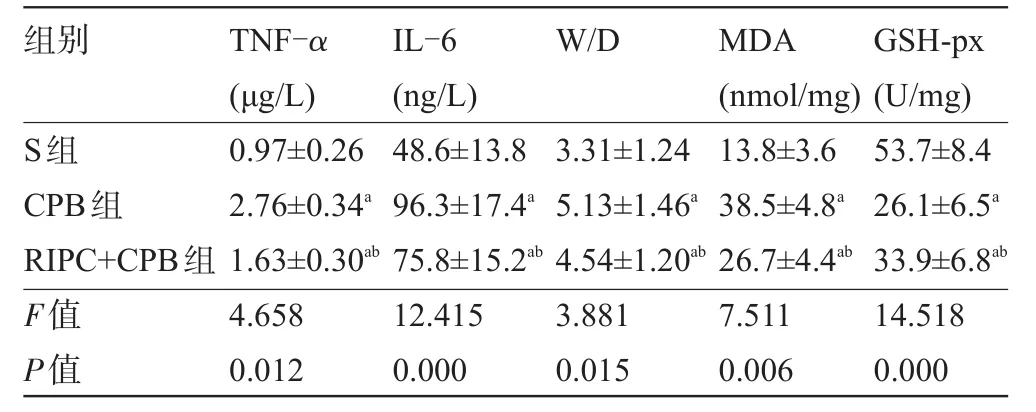
表1 三組大鼠血清TNF-α、IL-6、肺組織W/D比值、MDA和GSH-px活性比較(n=20,±s)
注:a與S組比較,P<0.05;b與CPB組比較,P<0.05。
F值P值14.518 0.000 4.658 0.012 12.415 0.000 3.881 0.015 7.511 0.006
3 討論
本研究采用尾動脈插管灌注,右頸靜脈插管引流的方法建立大鼠CPB模型,實現了接近生理的全流量轉流,并使用微型動物膜式氧合器,實現無血預充,從而模擬了臨床CPB的轉流過程,且各組大鼠均于轉流2 h后順利停機,并于自主呼吸恢復后拔除氣管導管,且存活,說明CPB模型制備成功。本研究結果顯示,大鼠CPB后肺組織W/D比值增加,肺間質出血、水腫,肺泡間隔增寬,肺泡腔內大量炎性細胞浸潤,提示CPB可造成一定程度的肺損傷,這與Chen等[5]的研究一致。
Dong等[6]研究表明,肺臟本身的缺血預處理可減輕肺組織的缺血再灌注損傷,但阻斷肺動脈可損傷血管內膜,且短暫缺血亦可造成肺上皮細胞損傷,因此限制了其臨床應用,而RIPC實施方便,對機體幾乎無損傷,可較好地避免上述缺陷[2]。本研究參照文獻[3]的方法,在大鼠CPB前實施RIPC干預,結果表明,與CPB組比較,RIPC+CPB組W/D比值下降,肺組織病理學改變減輕,提示RIPC可減輕大鼠CPB后的肺損傷。
目前所能檢測到的與CPB后肺損傷有明確關系的炎性因子主要包括TNF-α、IL-1β、IL-6、IL-8和IL-10[7-8]。其中TNF-α是主要的促炎因子,可通過多種途徑引起肺損傷;IL-6是炎性因子積聚的主要因素,并促進炎性因子進一步釋放,其水平與CPB炎性反應導致的組織損傷有密切關系。Goebel等[9]通過動物實驗證實,CPB后大鼠血清中TNF-α和IL-6水平即有增加,CPB結束后2 h達到高峰,本研究亦證實了這一觀點。而RICP干預后,可顯著降低血清中TNF-α和IL-6濃度,且肺組織W/D比值下降,肺組織病理學損傷減輕,提示RICP減輕大鼠CPB后肺損傷的機制,可能與抑制炎性反應有關。
MDA是脂質過氧化反應的產物,并間接反映組織受自由基攻擊的程度;GSH-px可特異的催化還原型谷胱甘肽對過氧化氫的還原反應,間接反映組織清除自由基的能力[10]。本研究結果顯示,大鼠CPB后肺組織MDA濃度增高,GSH-px活性下降,而實施RICP干預后,肺組織MDA濃度下降,GSH-px活性升高,且肺組織W/D比值下降,肺組織病理學損傷減輕,說明RICP可減少大鼠CPB后氧自由基的釋放,減輕肺組織細胞的損傷。
綜上所述,RIPC可減輕大鼠CPB后肺損傷,其機制可能與抑制炎性反應和脂質過氧化反應有關。但RIPC實施的最適時機和持續時間有待進一步研究。
[1]Young RW.Prevention of lung injury in cardiac surgery:a review [J].J Extra Corpor Technol,2014,46(2):130-141.
[2]符傳藝,王鵬程,趙建農,等.遠程缺血預處理的研究進展[J].海南醫學,2011,22(23):137-140.
[3]Hu X,Lu Y,Zhang Y,et al.Remote ischemic preconditioning improves spatial learning and memory ability after focal cerebral ischemia-reperfusion in rats[J].Perfusion,2013,28(6):546-551.
[4]Fujii Y,Shirai M,Tsuchimochi H,et al.Hyperoxic condition promotes an inflammatory response during cardiopulmonary bypass in a rat model[J].Artif Organs,2013,37(12):1034-1040.
[5]Chen S,Xu L,Tang J.Association of interleukin 18 gene polymorphism with susceptibility to the development of acute lung injury after cardiopulmonary bypass surgery[J].Tissue Antigens,2010,76 (3):245-249.
[6]Dong LY,Zheng JH,Qiu XX,et al.Ischemic preconditioning reduces deep hypothermic circulatory arrest cardiopulmonary bypass induced lung injury[J].Eur Rev Med Pharmacol Sci,2013,17(13):1789-1799.
[7]Luo Y,Wang Y,Poynter JA,et al.Pretreating mesenchymal stemcells with interleukin-1βand transforming growth factor-βsynergistically increases vascular endothelial growth factor production and improves mesenchymal stem cells-mediated myocardial protection after acute ischemia[J].Surgery,2012,151(3):353-363.
[8]楊志剛,張志新,李振東,等.烏司他丁對室間隔缺損手術患兒的肺氧合功能及C-反應蛋白、白介素-6水平的影響[J].海南醫學, 2013,24(22):3336-3338.
[9]Goebel U,Siepe M,Mecklenburg A,et al.Reduced pulmonary inflammatory response during cardiopulmonary bypass:effects of combined pulmonary perfusion and carbon monoxide inhalation[J]. Eur J Cardiothorac Surg,2008,34(6):1165-1172.
[10]Disli OM,Sarihan E,Colak MC,et al.Effects of molsidomine against doxorubicin-induced cardiotoxicity in rats[J].Eur Surg Res, 2013,51(1-2):79-90.
Protective effects of remote ischemic preconditioning on lung injury and its mechanism in rats undergoing cardiopulmonary bypass.
TU Jie,LIANG Dong-ke,LIU Guo-feng,LIANG Bei-wei,WEI Qiu-ying,LIU Ju-mei, ZHANG Bing-dong.Operation&Anesthesia Room,Institute of Cardiovascular Disease,the First Affiliated Hospital of Guangxi Medical University,Nanning 530021,Guangxi,CHINA
ObjectiveTo investigate the protective effects of remote ischemic preconditioning(RIPC)on lung injury and its mechanism in rats undergoing cardiopulmonary bypass(CPB).MethodsSixty adult male Sprague-Dawley rats were randomly divided into three groups(n=20 each):sham operation group(group S),group CPB and group RIPC+CPB.Rats in group S were anaesthetized and cannulated but did not undergoing CPB;rats in group CPB were cannulated in tail arteries and right jugular vein for CPB;rats in group RIPC+CPB were subjected to RIPC before CPB.RIPC was used by compressing the two hindlimbs alternately with a tourniquet for three cycles of 10 min ischaemia followed by 10 min reperfusion.The superior vena cava blood samples were collected at 2 h after anesthesia resuscitation for determination of concentrations of TNF-αand IL-6,and pulmonary tissue were obtained to observe the wet to dry weight ratio(W/D ratio),concentrations of malondialdehyde(MDA)and activity of glutathione peroxidase(GSH-px).The histopathological changes of lung tissue were also examined.ResultsCompared with group S,the plasma concentrations of TNF-αand IL-6,the W/D ratio and concentrations of MDA in lung tissues were increased,while activity of GSH-px in lung tissues was decreased in group CPB and group RIPC+CPB(P<0.05). Compared with group CPB,the plasma concentrations of TNF-αand IL-6,the W/D ratio and concentrations of MDA in lung tissues were decreased,while activity of GSH-px in lung tissues was increased in group RIPC+CPB(P<0.05). Compared with group CPB,the morphological injury of pulmonary tissue was alleviated in group RIPC+CPB under HE staining.ConclusionRIPC can reduce the lung injury in rats undergoing CPB.The mechanism is closely related to inhibiting the inflammatory reaction and lipid peroxidation.
Remote ischemic preconditioning;Cardiopulmonary bypass;Lung injury
R-332
A
1003—6350(2015)09—1256—03
10.3969/j.issn.1003-6350.2015.09.0452
2014-12-03)
廣西壯族自治區教育廳科學技術研究項目(編號:LX033)
張炳東。E-mail:zbdong2007@163.com