我院骨瓜提取物臨床應用及安全性回顧性評價
孔飛飛等
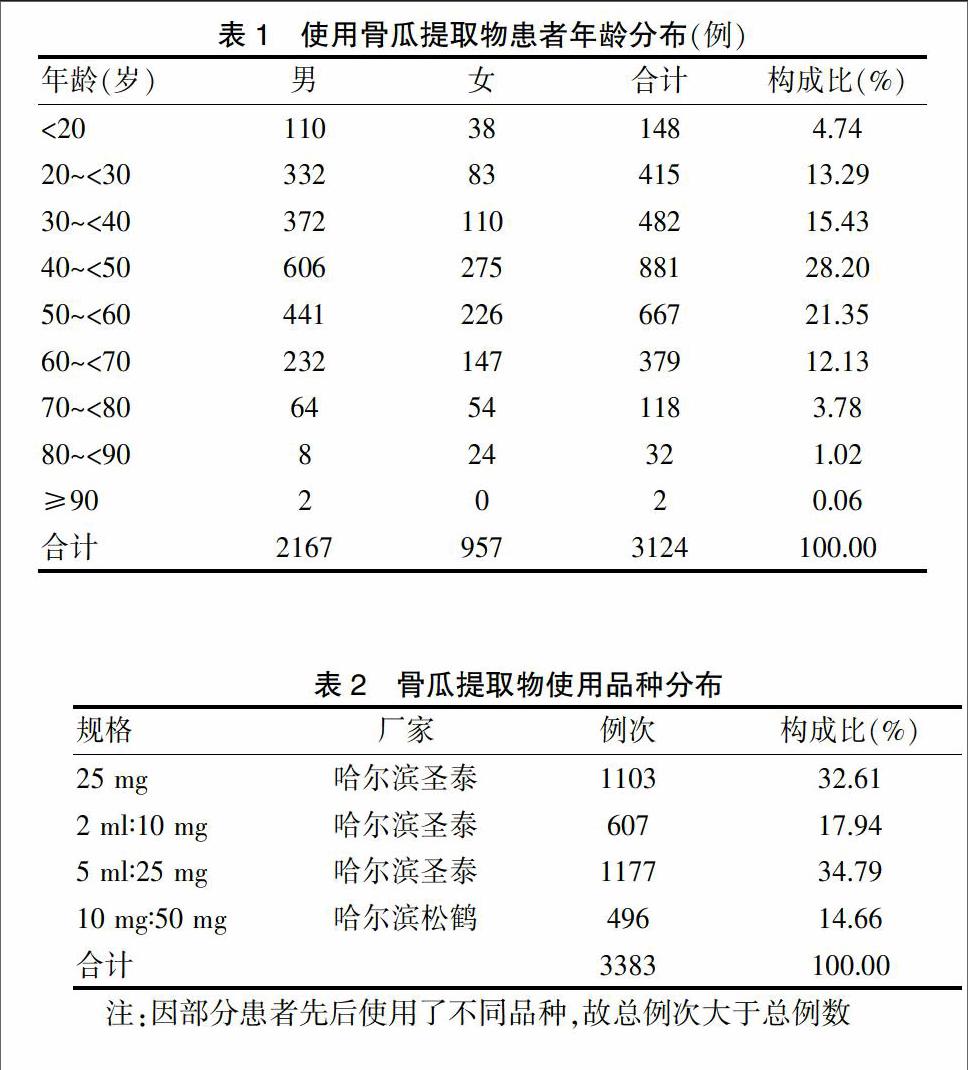
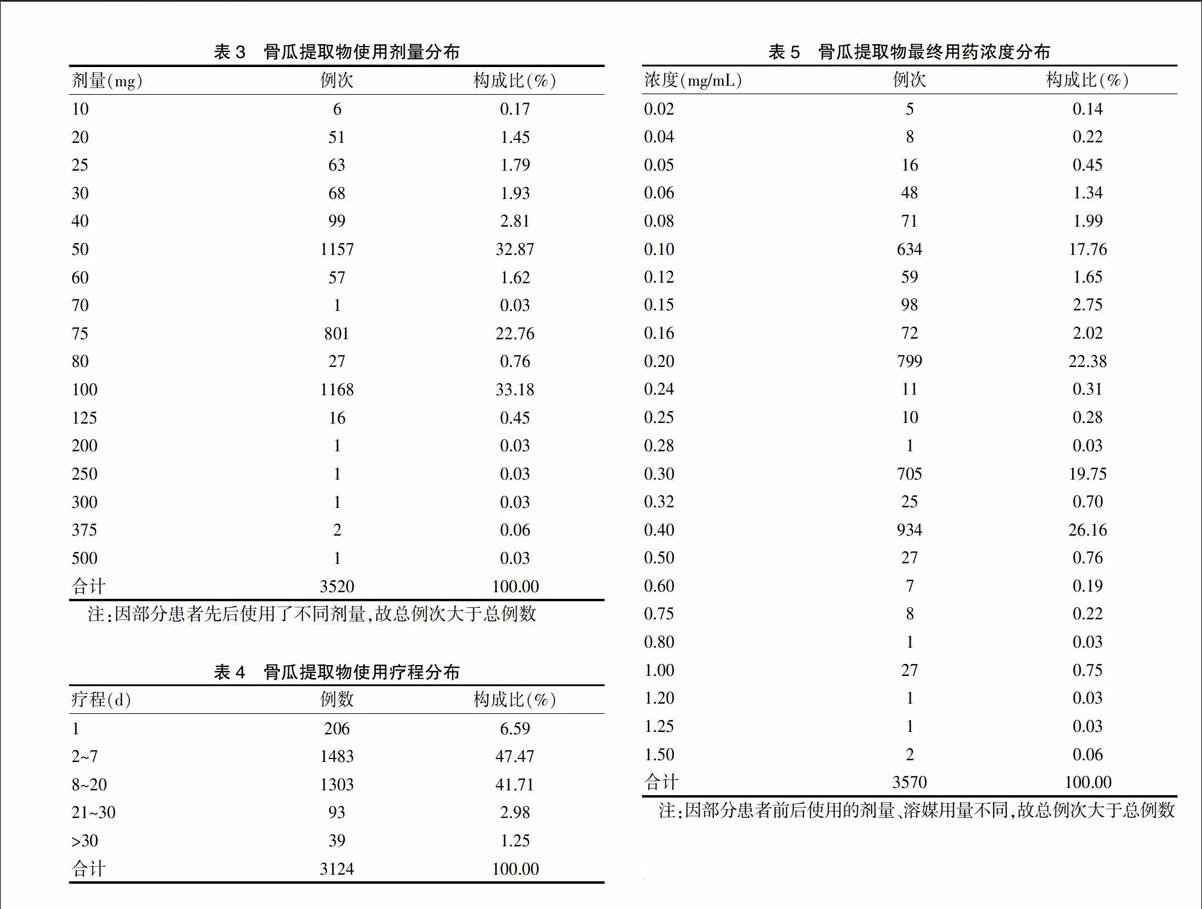
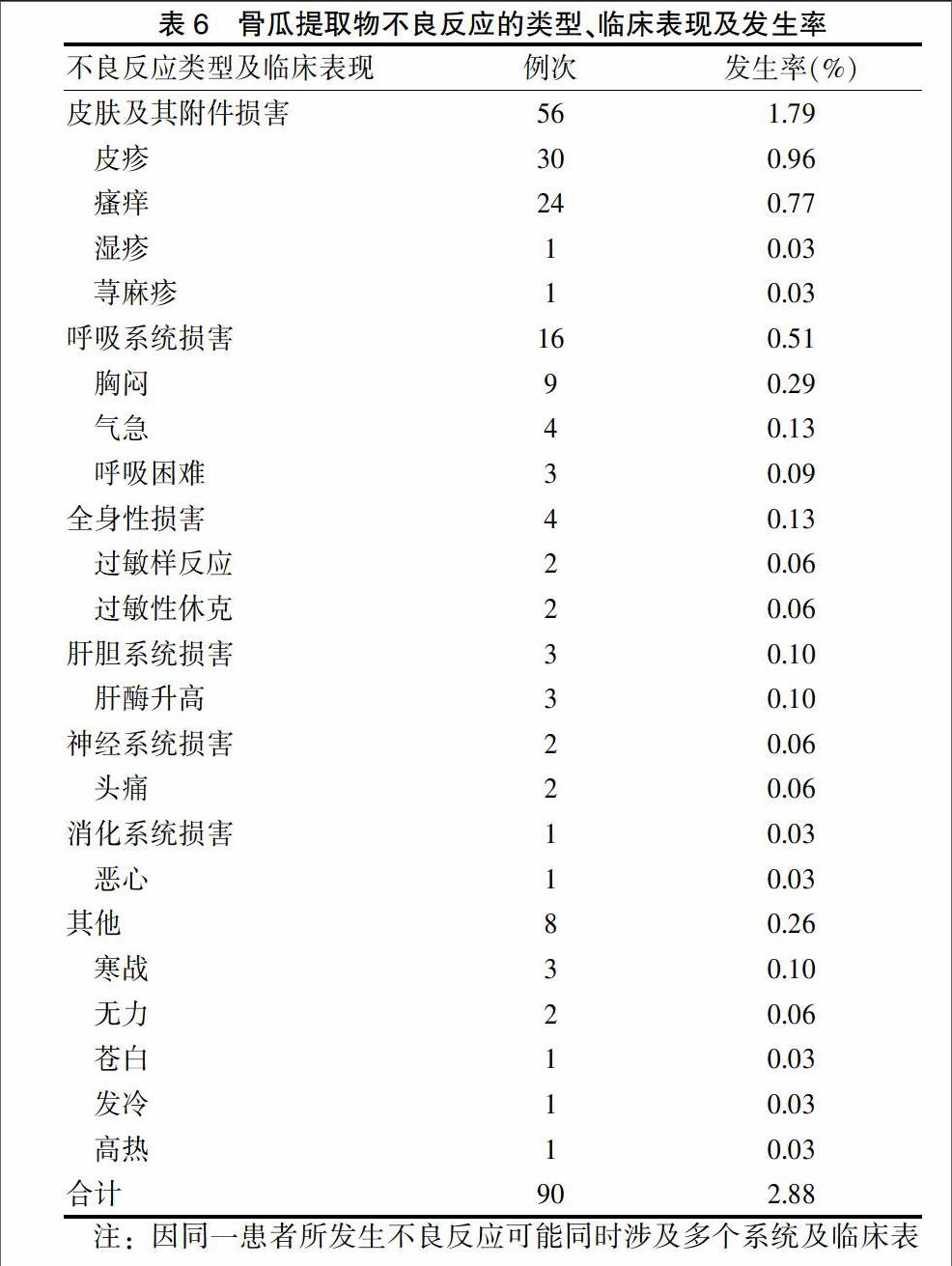
[摘要] 目的 分析解放軍第九八醫院(以下簡稱“我院”)骨瓜提取物應用情況及安全性,為臨床應用和藥品監管部門決策提供參考。 方法 采用病歷回顧性研究方法,對我院2010年10月~2012年12月期間使用骨瓜提取物的完整住院病歷進行調查,自建數據庫,進行統計分析。 結果 共收集到3124份應用骨瓜提取物的住院患者病歷,30~<60歲人群使用骨瓜提取物最多,占64.98%;適應證比較規范,用法均為靜脈滴注,用量、溶媒選擇、用藥濃度及用藥療程上存在超說明書現象;不良反應發生率為1.92%,屬常見范疇;主要表現為各種過敏反應,嚴重者可致過敏性休克,臨床表現多樣;累及全身多個系統,按發生率高低依次為皮膚及附件系統、呼吸系統、全身性、肝膽系統、神經系統、消化系統損害。 結論 骨瓜提取物臨床應用不良反應發生率較低,安全性較高;不合理應用給臨床用藥安全性增加了風險,應加強生化藥注射劑臨床合理使用監管。
[關鍵詞] 骨瓜提取物;臨床應用;安全性;回顧性;評價
[中圖分類號] R969.3 [文獻標識碼] A [文章編號] 1673-7210(2015)06(b)-0038-06
A retrospective evaluation on the clinical application and safety of Gugua Extract Injection
KONG Feifei1 WU Ying1 SHEN Jie2 ZHANG Yujie1 YE Guangming3 GUO Liangjun1 TAN Junming2
1.Department of Drug and Equipment, 98th Hospital of People's Liberation Army, Zhejiang Province, Huzhou 313000, China; 2.The 6th Department of Orthopaedics, 98th Hospital of People's Liberation Army, Zhejiang Province, Huzhou 313000, China; 3.Medical Department, 98th Hospital of People's Liberation Army, Zhejiang Province, Huzhou 313000, China
[Abstract] Objective To analyze the application and safety on Gugua Extract Injection in 98th Hospital of People's Liberation Army (“our hospital” for short), as references for proper clinical application and policy decisions from Drug Administration. Methods Cases on Gugua Extract Injection between October 2010 and December 2012 in our hospital were reviewed retrospectively and analyzed statistically on self-built database. Results In 3124 cases, 64.98% patients who took Gugua Extract Injection were aged 30-<60. Gugua Extract Injection was used under clinical indications by intravenous drip. In some cases, dosage, solvent, drug concentration and course of treatment were beyond specification. The incidence of ADRs was 1.92%; anaphylaxis was the main manifestations, severe cases could cause anaphylactic shock, diversity of clinical situation; ADRs involving multiple systems, included damage of skin and its appendages, respiratory system, systemic, hepatobiliary system, nervous system, digestive system. Conclusion The ADRs occurrence rate of Gugua Extract Injection is low, which has high security. Irrational clinical application can increase risk. The supervision of clinical application of biochemical drug injection should be strengthened.
[Key words] Gugua Extract Injection; Clinical application; Safety; Retrospective; Evaluation
骨瓜提取物是近年來應用于骨傷科的復方新藥制劑,主要用于風濕、類風濕關節炎、腰腿疼痛、骨折創傷修復等。目前對其的研究尚不多見,主要集中在骨瓜提取物單用或聯合其他治療手段的臨床療效觀察及細菌內毒素檢查方法等方面,亦有多篇骨瓜提取物致不良反應的病例報道[1],但對其在臨床特別是創傷骨科應用中的安全性尚未有客觀、系統的評價。解放軍第九八醫院(以下簡稱“我院”)擁有全軍創傷骨科中心,收治包括軍人、士兵、地方群眾在內的各種骨傷科病患,年收治病人達十萬人次,為促進創傷修復,多數會使用骨瓜提取物、骨肽、鹿瓜多肽等制劑。為進一步促進骨瓜提取物在臨床使用的安全、合理、有效,對其安全性進行一個客觀、系統的評價十分必要。因此,為了更進一步了解骨瓜提取物臨床應用的安全性,發現其不良反應規律,本研究采用病歷回顧性研究方法,開展病歷調查,對骨瓜提取物的不良反應和危險因素進行分析,為其在臨床的合理應用和藥品監管部門決策提供參考。

