堿性離子液體催化合成三羥甲基丙烷
龍金星 袁正求 馬 浩 舒日洋 李雪輝(中國科學院廣州能源研究所,中國科學院可再生能源重點實驗室,廣州50640;華南理工大學化學與化工學院,廣州50640)
堿性離子液體催化合成三羥甲基丙烷
龍金星1,*袁正求1馬浩2舒日洋1李雪輝2,*
(1中國科學院廣州能源研究所,中國科學院可再生能源重點實驗室,廣州510640;2華南理工大學化學與化工學院,廣州510640)
以堿性離子液體為催化劑,構建了一種新型、高效的合成三羥甲基丙烷(TMP)的方法.系統考察了催化劑類型、催化劑用量、反應溫度、反應時間、甲醛與正丁醛用量比等因素對三羥甲基丙烷分離收率的影響.研究結果表明:堿性離子液體的催化性能與其堿強度息息相關,堿性越強、催化活性越高.最優條件下,經離子液體[bmim]OH催化后,可得到84%的TMP分離收率,這一結果優于傳統的有機無機堿催化體系且成功規避了傳統堿催化合成TMP過程中繁瑣的除鹽過程、降低了過程能耗.此外,離子液體催化劑和未反應的丁醛均表現了良好的重復使用性能.
三羥甲基丙烷;離子液體;堿性強度;Cannizzaro縮合;重復使用性
www.whxb.pku.edu.cn
As a novel catalyst,ionic liquid(IL)combines the advantages of both homogenous and heterogeneous catalysts:high acid or alkali strength,uniform catalytic active centers,easy separation,and recyclability.5-7Therefore,ILs have been widely used in catalytic processes,such as material synthesis,organic catalysis,and more recent biomass conversion.8-15In 2005,Ranu and Banerjee16first reported that the Michael addition process could be promoted efficiently in the presence of the basic IL[bmim]OH. More than 90%product yield was achieved for all active methylene compounds examined.Inspired by this work,many basecatalyzed processes have been extensively investigated.17-21For example,Xu and coworkers18show that 73%-93%of product yield could be obtained during the Markovnikov addition reaction between alkyl-substituted imidazolium and the unsaturated ester with IL[bmim]OH catalyst.Yang et al.19also found that this functionalized IL shows much better catalytic activity than conventional inorganic and organic alkali catalysts in the aza-Michael addition reaction.Basic ILs demonstrate excellent activity for the Knoevenagel reaction and the Perkin reaction,as well.For instance,greater than 85%-95%substituted alkenes were produced at room temperature for reaction time of 10-30 min.21Recently,it has also been reported that this IL exhibits excellent catalytic performance in the facile and efficient synthesis of piperido 3',4':5,6 pyrano 2,3-d pyrimidinones20and the regioselective synthesis of thiazolidin-4-one derivatives.22However,few investigations focused on the TMP synthesis,a typical base-catalyzed process. In light of evidence supporting the excellent catalytic activity of basic IL on the abovementioned processes,we report a novel and efficient process for TMP production at mild reaction conditions utilizing these efficient ILs.
2 Experimental
2.1Materials
All of the reagents were analytical grade and used without further purification.1-Methyl imidazolium,1,2-dimethyl imidazolium,and 1-butyl bromide were purchased from Acros(Belgium).Formaldehyde,butyraldehyde,and other reagents were supplied by Tianjin Chemical Reagent Co.,Ltd.IL 1-butyl-3-methyl imidazolium hydroxyl([bmim]OH)was synthesized and purified according to the previously reported procedure.23ILs 1-butyl-3-methyl imidazolium formate([bmim]HCOO),1-butyl-3-methyl imidazolium acetate([bmim]Ac),1-butyl-2,3-dimethyl imidazolium acetate([bmmim]Ac),and 1-butyl-3-methyl imidazolium proline([bmim]Pro)were synthesized and their basicities were characterized according to our previous work.25The structures of these ILs were determined by Fourier transform infrared spectroscopy(FT-IR,Nicolet Nexus 870 FT-IR spectrometer (America),KBr coating),nuclear magnetic resonance(1H-NMR and13C-NMR,Mercury-Plus 400 MHz(America),D2O as solvent for[bmim]OH and d6-DMSO for others),and electrospray ionization mass spectrometry(ESI-MS,ThermoFinnigan LCQ Deca XP Plus(America)).NMR,ion chromatography,and elemental analysis24show that the purities of these ILs exceed 97%.
2.2TMP production,separation,and characterization
The TMP used in this study was obtained through consecutive processes ofAldol reaction and a Cannizzaro condensation.
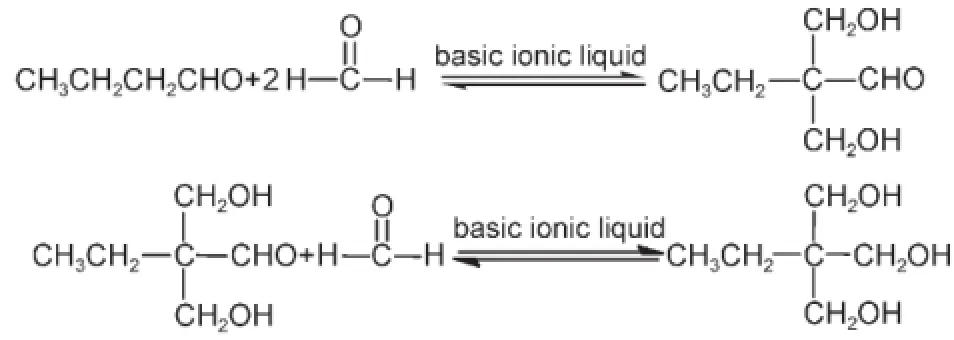
Typically,0.02 mol butyraldehyde,0.1 mol formaldehyde (measured as formaldehyde in 37%formalin),and a designated amount of basic IL catalyst were loaded into a 50 mL roundbottom flask.The mixture was stirred at 30°C for 2 h,and then stirred at 70°C for 4 h.When the stirring time elapsed,the mixture of reactant and product was separated according to the following procedure.
Formaldehyde was first removed by steam distillation.Next,free butyraldehyde was extracted from the water soluble fraction using toluene(20 mL×3).The large boiling point difference allowed the free butyraldehyde to be separated from toluene by distillation at reduced pressure.The coarse TMP product was collected from the water-soluble fraction by consecutively removing the water,CHCl3crystallization,and drying at 50°C for 3 h under reduced pressure.The resulting yellow solid was further recrystallized in ethyl acetate and dried at 50°C under reduced pressure until a constant mass was achieved.The final TMP product was obtained as a white solid.
The physical-chemical properties and purity of the final product were characterized by FT-IR(Nexus 870 FT-IR spectrometer,KBr pelleting),1H-NMR(Mercury-Plus 400 MHz,D2O),13C-NMR (Mercury-Plus 400 MHz,D2O),and melting point measurements (X-4 digital micro melting point apparatus).The yield of the final product was measured by comparing the mass of the isolated TMP to the theoretical value based on the initial quantity of butyraldehyde.
2.3Recyclability of lL
After the careful separation of free HCHO and HCOOH and after the removal of butyraldehyde by extraction using toluene,the IL catalyst was extracted from the water soluble fraction using CHCl3three times(10 mL×3).The recovered IL was obtained by the efficient removal of CHCl3at reduced pressure followed by drying at 70°C under vacuum overnight.It was then used for the next run without further purification.The anion concentrations of the original versus the used ILs were determined on a Metrohm 792 basic ion chromatograph(Switzerland)according to the previously reported procedure.24
3 Results and discussion
3.1Characterization of the basic lLs
All of the basic IL catalysts were intensively characterized by FT-IR,1H-NMR,13C-NMR,and ESI-MS(the results were listed as follows).Determination of basic strength using the acid-dissociation constant(pKb)shows that the basic strength sequence of these ILs25is[bmim]OH>[bmim]Pro>[bmmim]Ac>[bmim]Ac>[bmim]HCOO.
3.1.1[bmim]OH
FT-IR:3443,2958,2932,2873,1651,1601,1447,1394,1353,1122,871,778,694 cm-1.1H NMR(400 MHz,D2O)δ:8.38(s,1H),7.93(s,1H),7.88(s,1H),4.71(t,J=7.2 Hz,2H),3.27(s,3H),1.45(m,2H),1.21(m,2H),0.79(t,J=7.2 Hz,3H).13C NMR (400 Hz,D2O)δ:140.6,134.8,129.1,48.9,37.4,33.8,19.6,13.4. ESI-MS:positive ion mode,m/z=139(100%)[C8H15N2];negative ion mode,m/z=17(100%)[OH].
3.1.2[bmim]Pro
FT-IR:3443,2962,2871,2188,1579,1390,1169,1101,833,754,623 cm-1.1H NMR(400 Hz,DMSO)δ:9.54(s,1H),7.79(s,1H),7.71(s,1H),4.14(t,J=7.2 Hz,2H),3.83(s,3H),3.60(s,1H),3.12(s,1H),3.01(br,1H),2.87(m,1H),1.72(m,3H),1.57 (m,1H),1.45(m,1H),1.37(m,1H),1.21(q,2H),0.85(t,J=7.2 Hz,3H).13C NMR(400 Hz,DMSO)δ:177.0,137.6,124.0,122.7,63.1,48.9,47.5,36.1,31.9,31.6,26.5,19.2,13.7.ESI-MS:positive ion mode,m/z=139(100%)[C8H15N2];negative ion mode,m/z=114(100%)[C5H8NO2].
3.1.3[bmmim]Ac
FT-IR:3432,2962,2884,2133,1640,1572,1405,1347,1247,1124,1023,754,675 cm-1.1H NMR(400 MHz,DMSO)δ:7.86 (s,2H),4.15(t,J=7.2 Hz,2H),3.81(s,3H),3.12(s,3H),2.63(s,3H),1.68(m,2H),1.54(s,3H),1.27(m,2H),0.88(t,J=7.2 Hz,3H).13C NMR(400 MHz,DMSO)δ:173.4,144.6,122.9,121.5,49.0,47.3,34.8,31.6,19.1,13.7,9.3.ESI-MS:positive ion mode,m/z=153(100%)[C9H17N2];negative ion mode,m/z=60(100%)[C2H4O2].
3.1.4[bmim]Ac
FT-IR:3429,3151,3086,2957,2873,2186,1564,1467,1391,1326,1164,1008,918,762,645,613 cm-1.1H NMR(400 MHz,DMSO)δ:8.63(s,1H),7.97(s,1H),7.88(s,1H),4.20(t,J=7.2 Hz,2H),3.89(s,3H),1.77(m,2H),1.23(m,2H),0.85(t,J=7.2 Hz,3H).13C NMR(400 MHz,DMSO)δ:165.7,137.5,124.0, 122.8,48.6,36.0,34.8,32.0,19.2,13.6.ESI-MS:positive ion mode,m/z=139(100%)[C8H15N2];negative ion mode,m/z=60 (100%)[C2H4O2].
3.1.5 [bmim]HCOO
FT-IR:3443,2962,2884,2793,2715,2110,1639,1584,1471,1169,1113,766,652,632 cm-1.1H NMR(400 MHz,DMSO)δ:10.24(s,1H),8.62(s,1H),7.98(s,1H),7.86(s,1H),4.19(t,J= 7.2 Hz,3H),3.89(s,3H),1.75(m,2H),1.23(m,2H),0.86(t,J= 7.2 Hz,3H).13C NMR(400 MHz,DMSO)δ:173.8,138.3,124.0,122.7,48.5,31.5,26.5 19.5,13.9.ESI-MS:positive ion mode,m/z=139(100%)[C8H15N2];negative ion mode,m/z=45(100%)[CO2H].
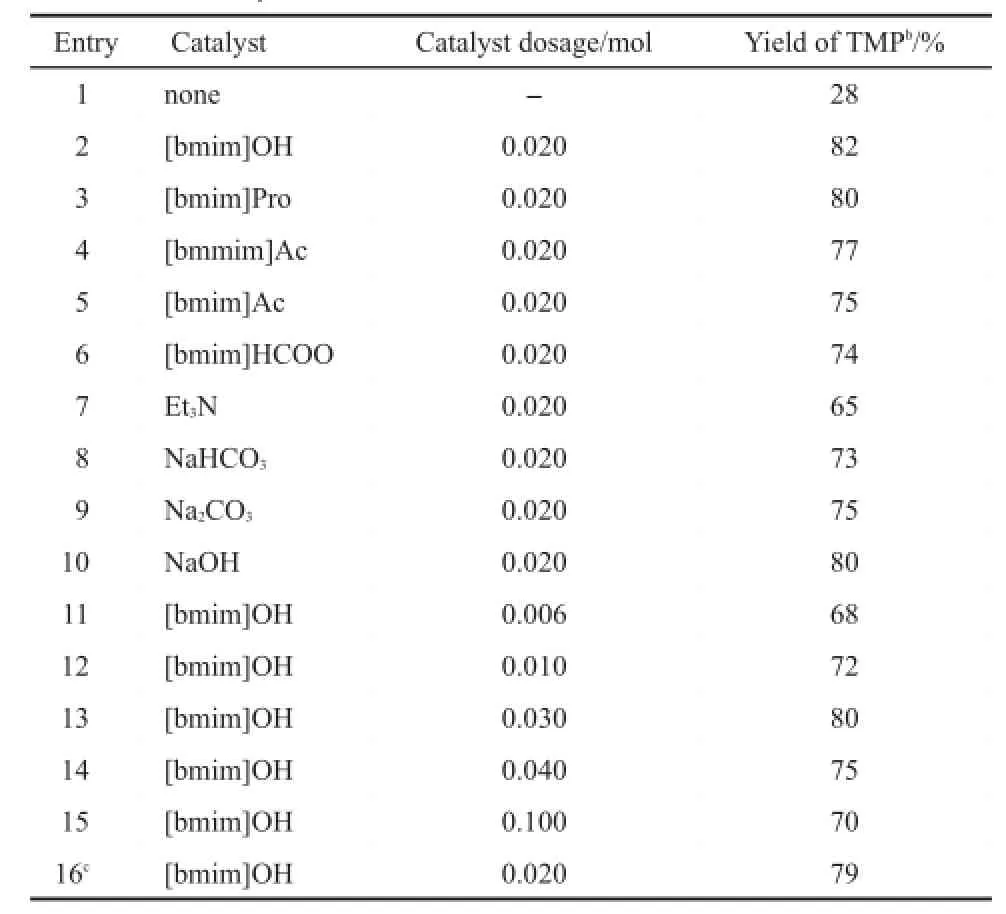
Table 1 Catalytic activities of basic ILs and conventional alkalisa
3.2Catalytic activities of lL catalysts
The catalytic performances of basic ILs are first investigated. Table 1 shows that TMP production yield is only 28%without the use of a catalyst.However,when a basic IL is present,74%to 82%isolated yield could be obtained.Generally,both the Aldol reaction and the Cannizarro reaction can be accelerated by alkali presence,hence,the addition of basic IL favors the production of TMP.Table 1 also demonstrates that the catalytic performances of the basic ILs are significantly influenced by their basic strengths,26where stronger bases result in higher catalytic activity.For example,[bmim]OH is the strongest basic IL and has the highest TMP yield,82%.According to the previous reports,27the TMP production process utilizes a carbanion mechanism.Thus,stronger basicity indicates a higher density of carbanions resulting in higher TMP yield.Further investigation demonstrates that[bmim]OH also exhibits much better catalytic performance than traditional organic and inorganic alkalis,such as NaOH,Na2CO3,NaHCO3,and Et3N.Moreover,compared with traditional alkalis,the desalination process is unnecessary for ILs.Therefore,ILs showgreat substitution potential for the traditional alkali catalyst in this process.
For homogeneous catalysts,larger catalyst dosages normally mean more active catalytic centers,resulting in higher catalytic performance.28-30Therefore,a sharp increase in TMP yield was observed with increasing catalyst dosage from 0.006 to 0.020 mol (Table 1).However,formaldehyde and butyraldehyde could catalytically condense with each other and themselves in the presence of[bmim]OH.For example,more than 76.8%conversion(determined by gas chromatography flame ionization detector (GC-FID)analysis)was observed when 0.04 mol butyraldehyde and 0.02 mol[bmim]OH were heated at 80°C for 5 h.Namely,during the TMP production,heteronuclear condensation of butyraldehyde with HCHO competes with self-condensation.Due to steric hindrance,the smaller molecule,HCHO,more readily attaches to the butyraldehyde molecule at low[bmim]OH concentrations.Nevertheless,when excess catalyst is present,the density of active carbanion intermediate of butyraldehyde increases significantly.As a result,both the heteronuclear condensation and self-condensation of butyraldehyde were promoted. Thus,the isolated yield of TMP was declined gradually when the added IL[bmim]OH was more than 2.0 mmol(Table 1).
3.3Effects of reaction temperature and time
Fig.1 shows the effects of reaction temperature and time on TMP production.As shown in Fig.1(a),TMP yield significantly increases when the Cannizzaro temperature is elevated from 50 to 70°C.It is well known that condensation could be accelerated significantly at high temperature,resulting in high conversion rates and product yields.30However,side reactions,such as dehydration and etherization of polyol,would also be accelerated. Therefore,no obvious increases in TMP yield are observed as temperature is increased above 70°C(Fig.1(a)).Reaction time is also a significant factor for TMP production.Fig.1b shows that TMP yield sharply increases from 70%to 80%when the reaction time is extended from 2 to 4 h.However,once thermodynamic equilibrium is reached(around 4 h),TMP yield becomes independent of reaction time.
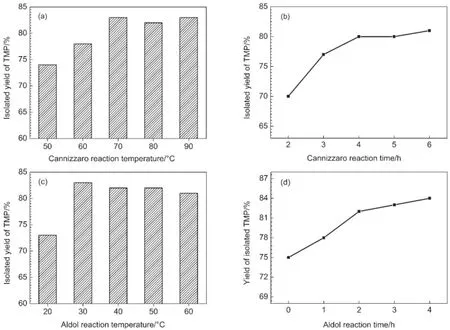
Fig.1 Effects of reaction temperature and time on the yield of TMP(a)Cannizzaro condensation temperature,(b)Cannizzaro condensation time,(c)Aldol reaction temperature,(d)Aldol reaction time.Reaction conditions are as follows:0.02 mol butyraldehyde,0.1 mol formaldehyde(measured as formaldehyde in 37%formalin),and 0.02 mol[bmim]OH.(a)Cannizarro time of 5 h,andAldol temperature of 30°C for 1 h;(b)Cannizarro temperature of 70°C,andAldol temperature of 30°C for 1 h;(c)Cannizarro temperature of 70°C for 4 h,andAldol time of 1 h;(d)Cannizarro temperature of 70°C for 4 h,andAldol temperature of 30°C
The influences of Aldol reaction temperature and time on the isolated yield of TMP were also studied(Fig.1(c,d)).Fig.1(c)demonstrates how the TMP yield increases sharply(73%to 83%)when the reaction temperature is increased from 20 to 30°C,and then plateaus upon increasing the temperature further.Fig.1(d)shows that the isolated yield of TMP is significantly influenced by the Aldol reaction time.The isolated yield of TMP gradually increases with prolonged reaction time.For example,less than 75% TMP yield is achieved without the Aldol process,but sharply increases to 82%when the reactants and catalyst are pretreated at 30°C for 2 h(Fig.1(d)),suggesting that the Aldol condensation process is essential for efficient TMP production.However,increases in TMP yield are less significant at Aldol reaction timesgreater than 2 h.Hence,2 h is considered to be an appropriate reaction time for Aldol condensation.In light of the abovementioned discussion,we can safely conclude that TMP production is temperature dependent.Compared with the Cannizzaro condensation,theAldol reaction is much faster at any given temperature. Thus,the Cannizzaro condensation can be considered a stepcontrolled process for TMP production.
3.4Effect of dosage ratio of formaldehyde and
butyraldehyde
Reactant dosage ratio of formaldehyde to butyraldehyde was also examined.Fig.2(a)shows that TMP yield first dramatically increases and then gradually decreases,attaining a maximum at the reactant dosage ratio of 6:1.For example,TMP yield increases from 50%to 84%when the dosage ratio of formaldehyde to butyraldehyde is increased from 3:1 to 6:1.Yield then decreased to 75%when the feed of HCHO grew to ten times that of butyraldehyde.In our opinion,the dramatic increase can be attributed to the following:(1)increased reactant promotes heteronuclear condensation,and(2)dilution with the 37%HCHO solution inhibits self-condensation of butyraldehyde.However,too much HCHO solution causes a concentration reduction of both [bmim]OH and butyraldehyde,reducing TMP yield(Fig.2(a)).
3.5Recyclability of lL and butyraldehyde
Recovery of the IL involves careful separation and removal of CHCl3followed by drying at 70°C under vacuum overnight.Our results demonstrate that the IL catalyst is recyclable,achieving a TMP yield of 79%at optimized conditions after three runs(see the last entry of Table 1).The reduction of TMP yield cannot be attributed to the thermal decomposition of the IL catalyst,according to previous literature.19,21The reduction is more likely attributed to weight loss during the separation process(10.6% weight loss occurs after three runs).Additionally,12.5%of the OH-groups are replaced by HCOO-(measured by ion chromatography)after three runs.This change also contributes to a reduction in catalytic activity since[bmim]HCOO demonstrates lower catalytic activity than[bmim]OH(Table 1).
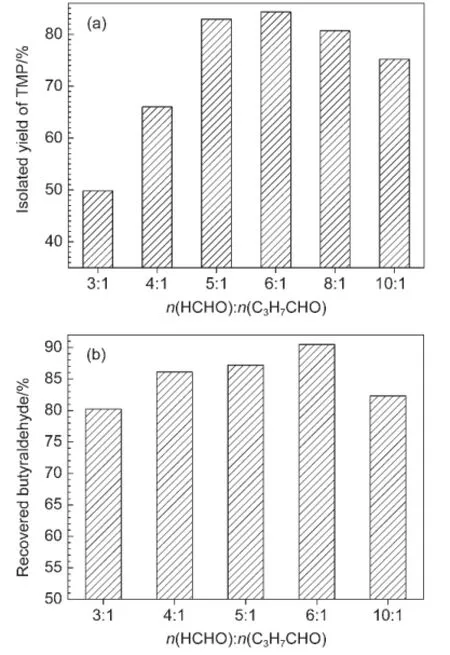
Fig.2 (a)Effects of molar ratio of the feedstock on TMPyield,and(b)the recyclability of free butyraldehydeReaction conditions are as follows:Aldol temperature of 30°C for 2 h,and Cannizarro temperature of 70°C for 4 h during reactions containing(a)0.02 mol butyraldehyde,and(b)0.2 mol butyraldehyde.
Butyraldehyde is an important intermediate and feedstock for the fine chemical industry.Furthermore,this colorless liquid has an acrid smell and is flammable.32Therefore,recycling butyraldehyde is necessary.Fig.2(b)shows that this chemical is recyclable,where greater than 80%of unreacted butyraldehyde can be successfully extracted by toluene and separated by distillation. Fig.2(b)also demonstrates that the extraction of this compound dramatically increases up to 90.7%when the molar ratio of HCHO to butyraldehyde is 6:1,followed by a drop-off in recyclability when the formaldehyde dosage ratio reaches 10:1.At low formaldehyde doses,not only is TMP produced,but also butyraldehyde self-condenses(76.8%conversion during the selfcondensation process with[bmim]OH),as discussed above. However,a formaldehyde concentration that is too high dilutes the butyraldehyde,resulting in a reduction of extraction efficiency.
3.6Characterization of the final product
Fig.3 shows the1H-NMR spectrum of the final TMP product. The triplet peak at δ 0.79(3H)is attributed to the methyl group of TMP.The chemical shifts at 1.24(2H)and 3.44(6H)are ascribed to the CH2groups adjacent to CH3and OH,respectively.The hydroxyl shows a single peak at δ 4.70(3H).
The13C-NMR spectrum of the final TMP product was also investigated.The results shown in Fig.4 demonstrate that there are four carbons in the TMP molecule.The signal at 6.47 is assigned to CH3.The peak at 20.95 is attributed to the absorption from CH2. Quaternary carbon shows a signal peak at 43.22.The absorption peak at 62.13 is assigned to the carbon atom in CH2OH.Both the1H-NMR and the13C-NMR spectra of our produced TMP show similar signals to the standard spectra of TMP,and they agree well with previously reported studies.1,26
FT-IR spectra were collected to further characterize the final TMP products(Fig.5).The produced TMP shows a similar absorption spectrum to the standard TMP spectrum.The absorbance at 3334 cm-1is considered to be the characteristic absorption of hydroxyls.33The peak at 1489 cm-1suggests the existence of the primary alcohol(CH2OH).The absorbencies at 2934,2910,and 2858 cm-1are designated as the stretching vibrations of―CH2OH,CH,and CH2,respectively.34The absorbencies at 1376,1292,1193,and 782 cm-1are characteristic peaks of alkyl groups,CH3,CH2,and CH.35The characteristic absorption peaks at 1064 and 1058 cm-1are ascribed to the C―O stretching vibration of primary alcohol.Noticeably,the characteristic infrared absorption ofC=O,which generally ranges from 1750 to 1700 cm-1,is not present.In light of these findings,in conjunction with the1HNMR(Fig.3)and13C-NMR(Fig.4)results,we can safely conclude that the TMP produced by this basic IL catalytic process has high purity.Furthermore,the final TMP product has a narrow melting point between 58 and 60°C,in good agreement with previously reported melting points of TMP.1,26
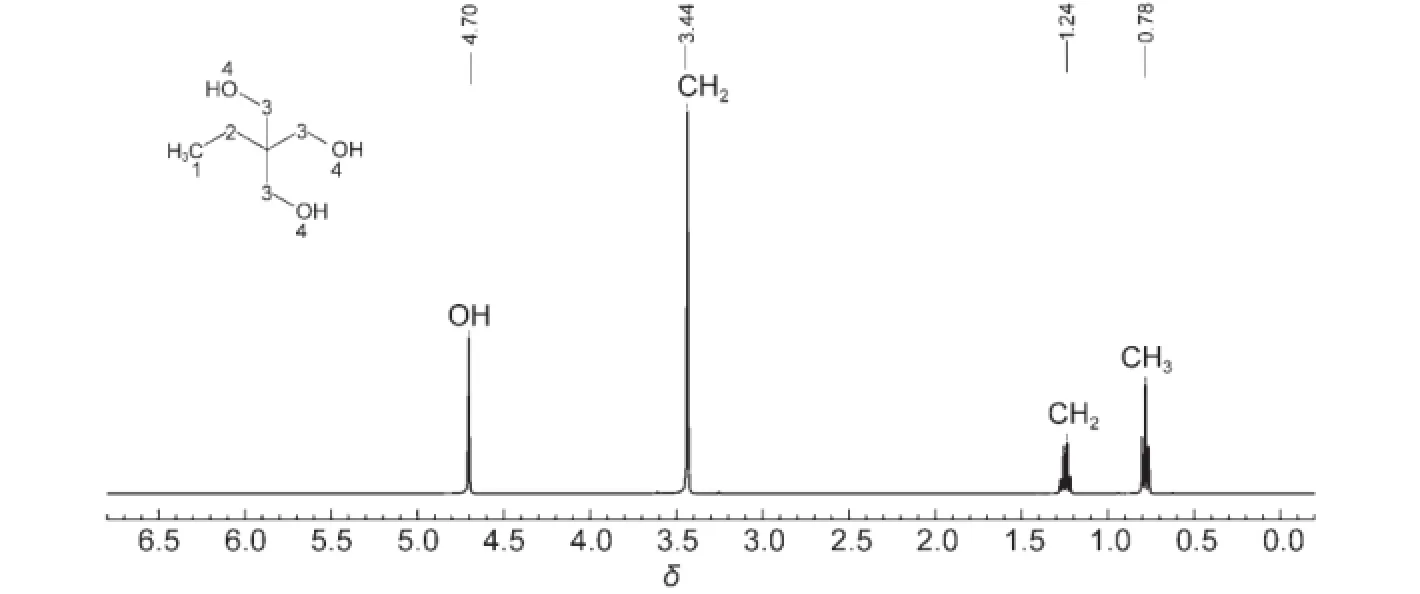
Fig.3 1H-NMR spectrum of the final TMPproduct

Fig.4 13C-NMR spectrum of the final TMPproduct
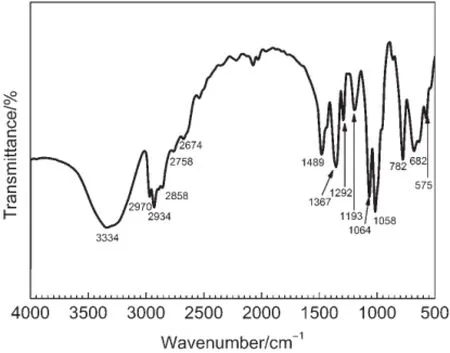
Fig.5 FT-IR spectrum of the final TMPproduct
4 Conclusions
In conclusion,an efficient and novel strategy for producing TMP is proposed using basic IL catalysts.The catalytic performance of the ILsignificantly depends on the basic strength,where higher basic strength results in higher catalytic activity.Under optimized conditions,isolated yields greater than 84%are attainable with the strongest basic IL,[bmim]OH.This yield was higher than that attained by conventional alkalis.Furthermore,both the[bmim]OH and the butyraldehyde demonstrate good recyclability,after which,the IL showed slight activity loss(after three runs)while 90.7%of free butyraldehyde could be recovered. Moreover,this method has the advantage of avoiding the tedious and labor-intensive desalination process,which is necessary for traditional base catalytic processes.Therefore,this efficient process provides a promising and beneficial method for future production of TMP.
References
(1)Werle,P.;Morawietz,M.;Lundmark,S.;Sorensen,K.;Karvinen,E.;Lehtonen,J.Alcohols,Polyhydric.In Ullmann?s Encyclopedia of Industrial Chemistry;Wiley-VCH Verlag GmbH&Co.KGaA:Weinheim,Germany,2008.
(2)Serra-Holm,V.;Salmi,T.;Multam?ki,J.;Reinik,J.;M?ki-Arvela,P.;Sjoholm,R.;Lindfors,L.P.Appl.Catal.A:Gen. 2000,198(1-2),207.
(3)Rantakyl?,T.K.;Salmi,T.;Aumo,J.;M?ki-Arvela,P.;Sjoholm,R.;Ollonqvist,T.;V?yrynen,J.;Lindfors,L.P.Ind.Eng.Chem. Res.2000,39(8),2876.doi:10.1021/ie990745h
(4)Rantakyl?,T.K.;Salmi,T.;Kuusisto,J.;M?ki-Arvela,P.;Ollonqvist,T.;V?yrynen,J.;Lindfors,L.P.Ind.Eng.Chem.Res.2002,41(3),524.doi:10.1021/ie001000a
(5)Zhang,Q.;Zhang,S.;Deng,Y.Green Chem.2011,13(10),2619.doi:10.1039/c1gc15334j
(6)Ning,H.;Hou,M.Q.;Yang,D.Z.;Kang,X.C.;Han,B.X. Acta Phys.-Chim.Sin.2013,29(10),2107.[寧匯,侯民強,楊德重,康欣晨,韓布興.物理化學學報,2013,29(10),2107.]doi:10.3866/PKU.WHXB201304172
(7)Yuan,X.;Lou,S.J.;Kou Y.Acta Phys.-Chim.Sin.2010,26(4),908.[苑曉,婁舒杰,寇元.物理化學學報,2010,26(4),908.]doi:10.3866/PKU.WHXB20100433
(8)Pinkert,A.;Marsh,K.N.;Pang,S.S.;Staiger,M.P.Chem.Rev. 2009,109(12),6712.doi:10.1021/cr9001947
(9)Gong,Y.Y.;Liu,M.;Jia,S.Y.;Feng,J.P.;Song,C.S.;Guo,X. W.Acta Phys.-Chim.Sin.2012,28(3),686.[公艷艷,劉民,賈松巖,馮建萍,宋春山,郭新聞.物理化學學報,2012,28(3),686.]doi:10.3866/PKU.WHXB201112292
(10)Zakrzewska,M.E.;Bogel-Lukasik,E.;Bogel-Lukasik,R. Chem.Rev.2011,111(2),397.doi:10.1021/cr100171a
(11)Parvulescu,V.I.;Hardacre,C.Chem.Rev.2007,107(6),2615.doi:10.1021/cr050948h
(12)Long,J.;Li,X.;Wang,L.;Zhang,N.Sci.Chin.Chem.2012,55 (8),1500.doi:10.1007/s11426-012-4633-7
(13)Li,Z.;Zhao,Y.W.;Han,F.;Yang,L.;Song,H.Y.;Chen,J.;Xia,G.C.Sci.Sin.Chim.2012,42(4),502.[李臻,趙應偉,韓峰,楊磊,宋河遠,陳靜,夏春谷.中國科學:化學,2012,42(4),502.]doi:10.1360/032011-727
(14)Zhu,Y.H.;Zeng,H.Y.;Li,S.S.;Lu,Z.X.;Ma,C.A.Acta Phys.-Chim.Sin.2012,28(2),421.[朱英紅,曾紅燕,李珊珊,陸在祥,馬淳安.物理化學學報,2012,28(2),421.]doi:10.3866/PKU.WHXB201112122
(15)Hallett,J.P.;Welton,T.Chem.Rev.2011,111(5),3508.doi:10.1021/cr1003248
(17)Moreira,D.N.;Longhi,K.;Frizzo,C.P.;Bonacorso,G.H.;Zanatta,N.;Martins,A.M.Catal.Commun.2010,11,476.doi:10.1016/j.catcom.2009.12.001
(18)Xu,J.;Liu,B.;Wu,W.;Qian,C.;Wu,Q.;Lin,X.J.Org.Chem. 2006,71,3991.doi:10.1021/jo0600914
(19)Yang,L.;Xu,L.;Zhou,W.;Li,L.;Xia,C.Tetrahedron Lett. 2006,47,7723.doi:10.1016/j.tetlet.2006.08.103
(20)Siddiqui,I.R.;Srivastava,A.;Shamim,S.;Srivastava,A.;Rahila,M.A.W.S.;Abumhdi,A.A.H.;Srivastava,A.;Rai,P. J.Mol.Catal.A Chem.2014,382,126.doi:10.1016/j. molcata.2013.10.026
(21)Li,J.;Sun,H.;Cai,X.C.;Dai,L.Y.Chin.J.Org.Chem.2007,27,1296.[李娟,孫輝,蔡曉晨,戴立益.有機化學,2007,27,1296.
(22)Mamaghani,M.;Pourranjbar,M.;Nia,R.H.J.Sulf.Chem. 2014,35(1),1.doi:10.1080/17415993.2013.800061
(23)Mehnert,C.P.;Dispenziere,N.C.;Cok,R.A.Chem.Commun. 2002,2,1601.
(24)Li,X.H.;Duan,H.L.;Pan,T.J.;Wang,L.F.Chin.J.Anal. Chem.2006,34,S192.[李雪輝,段紅麗,潘景添,王樂夫.分析化學,2006,34,S192.]
(25)Chen,X.W.;Song,H.B.;Chen,P.;Wang,F.R.;Qian,Y.;Li,X. H.Acta.Chim.Sin.2012,70,770.[陳學偉,宋紅兵,陳鵬,王芙蓉,錢宇,李雪輝.化學學報,2012,70,770.]doi:10.6023/A1108223
(26)Abd Hamid,H.;Yunus,R.;Rashid,U.;Choong,S.T.;Al-Muhtaseb,H.A.Chem.Eng.J.2012,200,532.
(27)Smith,B.M.;March,J.March?s Advanced Organic Chemistry;John Wiley&Sons,2009.
(28)Long,J.;Guo,B.;Teng,J.;Yu,Y.;Wang,L.;Li,X.Bioresour. Technol.2011,102,10114.doi:10.1016/j.biortech.2011.08.043
(29)Zhao,Y.;Long,J.;Deng,F.;Xia,C.;Peng,J.Catal.Commun. 2009,10,732.doi:10.1016/j.catcom.2008.11.030
(30)Long,J.X.;Guo,B.;Li,X.H.;Wang,F.R.;Wang,L.F.Acta Phys.-Chim.Sin.2011,27(5),995.[龍金星,郭斌,李雪輝,王芙蓉,王樂夫.物理化學學報,2011,27(5),995.]doi:10.3866/PKU.WHXB20110506
(31)Clayden,J.;Greeves,N.;Warren,S.Organic Chemistry,2nd ed.;Oxford University Press:London,2012.
(32)Cornils,B.;Fischer,R.W.;Kohlpaintner,C.Butanals,In:Ullmann?s Encyclopedia of Industrial Chemistry;Wiley-VCH Verlag GmbH&Co.KGaA:Weinheim,Germany,2000.
(33)Long,J.;Zhang,Q.;Wang,T.;Zhang,X.;Xu,Y.;Ma,L. Bioresour Technol.2014,154,10.doi:10.1016/j. biortech.2013.12.020
(34)Mayo,W.D.;Miller,A.F.;Hannah,W.R.Course Notes on the Interpretation of Infrared and Raman Spectra;John Wiley& Sons:New Jersey,2004.
(35)Silverstein,R.M.;Webster,F.X.;Kiemle,J.D.Spectrometric Identification of Organic Compounds,7th ed.;John Wiley& Sons:Westford,USA,2005.
Catalytic Synthesis of Trimethylolpropane in the Presence of Basic lonic Liquid
LONG Jin-Xing1,*YUAN Zheng-Qiu1MAHao2SHU Ri-Yang1LI Xue-Hui2,*
(1Key Laboratory of Renewable Energy,Guangzhou Institute of Energy Conversion,Chinese Academy of Sciences,Guangzhou 510640,P.R.China;2School of Chemistry and Chemical Engineering,South China University of Technology,Guangzhou 510640,P.R.China)
Ionic liquid(IL)catalyst attracts increasing attention during last few decades due to its excellent advantages of both homogeneous and heterogeneous catalyst.Here,a novel and efficient strategy for the production of trimethylolpropane(TMP)is proposed with basic IL catalysts.The catalytic activities of the IL catalysts are investigated.The effects of catalyst dosage,reaction temperature,time,and the molar ratio of the reactants are also studied.The results show that the basicity of the IL catalyst has a significant effect on the catalytic activity,where stronger basicity indicates higher catalytic activity.The IL 1-butyl-3-methyl imidazolium hydroxyl([bmim]OH)is proven to be the most efficient catalyst where more than 84%isolated yield of TMP was obtained under optimized conditions.Furthermore,both the IL catalyst and butyraldehyde show excellent recyclability.Moreover,this IL catalytic system avoids the inevitable desalination encountered by current processes utilizing inorganic alkali catalysts.
Trimethylolpropane;Ionic liquid;Basic strength;Cannizzaro condensation;Recyclability
1 lntroduction
Trimethylolpropane(TMP),a polyol containing α-hydroxymethyl and neopentyl functional groups,has been regarded as an important intermediate in the organics industry.1Generally,TMPis synthesized via a two-step process,namely,an Aldol condensation of formaldehyde and butyraldehyde followed by a Cannizzaro condensation2or catalytic hydrogenation.3,4The catalytic hydrogenation method usually suffers from high reaction temperatures and high H2pressure,requiring robust equipment for the dangerous operating conditions.In contrast,the Cannizzaro condensation is more popular because of its gentle reaction conditions and efficient product separation.Conventional alkalis,such as NaOH,KOH,Ca(OH)2,and Na2CO3,were proven to be efficient catalysts for this process,and 70%-80%yields have been demonstrated.1,2However,the homogeneous catalysts are corrosive and difficult to recover.Heterogeneously catalyzed processes generally suffer from high catalyst/substrate molar ratios and unevenly distributed active centers.5Furthermore,the post-separation and product purifaction are tedious and laborintensive(especially desalination).
September 30,2014;Revised:December 10,2014;Published on Web:December 10,2014.
O643
10.3866/PKU.WHXB201412101
The project was supported by the National Natural Science Foundation of China(51306191,21336002)and Doctoral Fund of Ministry of Education of China(20130172110043).
國家自然科學基金(51306191,21336002)及教育部博士點基金(20130172110043)資助項目
?Editorial office ofActa Physico-Chimica Sinica

