基于5-磺酸基間苯二甲酸鈉和雙三唑烷烴的Cu配合物的合成、結構和性質
李 婷 李 欣 周尚永 田 麗
(天津師范大學化學學院,天津市功能分子結構與性能重點實驗室,無機-有機雜化功能材料化學省部共建教育部重點實驗室,天津300387)
李婷李欣周尚永田麗*
(天津師范大學化學學院,天津市功能分子結構與性能重點實驗室,無機-有機雜化功能材料化學省部共建教育部重點實驗室,天津300387)
利用5-磺酸基間苯二甲酸鈉(NaH2sip)和雙三唑烷烴,合成了3個配合物[Cu0.5(btm)(H2O)](H2sip)·H2O}n(1,btm=雙三唑甲烷),{[Cu(btp)2(H2sip)(H2O)](NO3)·4H2O}n(2,btp=雙三唑丙烷)和{[Cu(btb)2(Hsip)]n(3,btb=雙三唑丁烷)。化合物1為一維雙鏈結構;化合物2為二維四方網絡結構,多個二維層依次疊加形成三維超分子結構;化合物3也具有二維層狀結構,其中金屬銅離子和雙三唑丁烷構成的一維雙鏈結構經雙齒μ2-Hsip2-配體連接構成了二維層狀結構。同時對配合物的熱穩定性和順磁共振特性進行了討論。
雙三唑甲烷;雙三唑丙烷;雙三唑丁烷;5-磺酸基間苯二甲酸單鈉鹽;超分子結構;氫鍵
Coordinationpolymershaverecentlyaroused much interest as materials,due to not only the structural diversity but also their attractive properties, such as catalytic activity,magnetism,photochemical activity and electrical chemistry[1-9].One of the key steps for preparation of polymeric transition metalcomplexesistoselectthemultidentatebridging ligands or mixed coordination ligands[10-14].Currently, the rational construction of new structurally defined MOFs using the mixed-ligand strategy seems to be a marvelous success[15-21].
Because of the diversity of the coordination modes and high structural stability,multi-carboxylic ligands with suitable spacers,especially benzoic acid based ligands are frequently used for metal-organic networks[22-27].Benzene-1,3,5-tricarboxylic acid(H3btc, also known as trimesic acid)is a rigid,planar molecule and has been widely used as a bridging ligand in the synthesis of multidimensional MOFs.Compared to the widely used benzene-1,3,5-tricarboxylic acid,5-sulfoisophthalic acid monosodium salt(NaH2sip)has distinctive characteristics:(i)C2symmetry of the ligand may cause the generation of different structures;(ii)the sulfonate group is generally perceived as a weaker group with respect to their coordinating ability and has one more potentially coordinating oxygen atom; (iii)the weak coordination nature of-SO3makes its coordination mode very flexible and sensitive to the chemical environment.
On the other hand,bis(1,2,4-triazol-1-yl)alkanes arehighlyflexibleligands.Theflexibilityand conformation freedoms of bistriazole alkanes can offer the possibility for the construction of unpredictable and interesting frameworks.
In this contribution,we describe a series of Cumetal-organic frameworks constructed from rigid multi -carboxylic ligand NaH2sip and flexible bistriazole alkanes.Three novelcomplexes[Cu0.5(btm)(H2O)] (H2sip)·H2O}n(1),{[Cu(btp)2(H2sip)(H2O)](NO3)4H2O}n(2),and{[Cu(btb)2(Hsip)]n(3)were fabricated and structurallycharacterizedbyX-raysinglecrystal analyses.They exhibited novel framework structures varying from 1D chains,to 2D layers.The thermal stability and EPR spectra have also been discussed.
1 Experimental
1.1General considerations
Thereagentsandsolventsemployedwere commercially available and used as received without further purification.Bis(1,2,4-triazol-1-yl)alkanes was synthesized as reported previously[28].The elemental analyses(C,H,and N)were carried out on a Perkin-Elmerelementalanalyzer.TGexperimentswere performed on a NETZSCH TG 209 instrument with a heating rate of 10℃·min-1under nitrogen conditions. EPR spectra were measured on a BRUKER EMX-6/1 EPR spectrometer.
1.2Preparation
[Cu0.5(btm)(H2O)](H2sip)·H2O}n(1).A mixture of Cu(NO3)2·3H2O(168 mg,0.7 mmol),NaH2sip(189 mg, 0.7 mmol),btm(91 mg,0.7 mmol)and H2O(12 mL) was added into a parr Teflon-lined stainless steel vessel(25 mL),and then the vessel was sealed and heated to 140℃.After 3 days the autoclave was cooled to room temperature at a rate of 1.5℃·h-1. Blue crystalline products 1 were filtered off,washed with distilled water and dried in air.Yield:45% (based on Cu).Anal.Calcd.(%)for C13H15Cu0.5N6O9S (463.15):C 33.71,H 3.26,N 18.15.Found(%):C 33.38,H 3.55,N 18.46.
{[Cu(btp)2(H2sip)(H2O)](NO3)·4H2O}n(2).Blue crystals of 2 were obtained by adopting the similar synthetic procedure as 1 except that btm was replaced by btp(111 mg,0.7 mmol).Yield:48%(based on Cu).Anal.Calcd.(%)for C22H35CuN13O15S(817.25):C 32.33,H 4.32,N 22.29.Found(%):C 32.65,H 4.37, N 22.54.
[Cu(btb)2(Hsip)]n(3).Bluecrystalsof 3 were obtained by adopting the similar synthetic procedure as 1 except that btm was replaced by btb(120 mg,0.7 mmol).Yield:43%(based on Cu).Anal.Calcd.(%)for C12H14Cu0.5N6O3.5S0.5(346.10):C 41.64,H 4.08,N 24.29. Found(%):C 41.35,H 4.18,N 24.55.
1.3X-ray crystallography
Single-crystal X-ray diffraction measurements of 1~3 were carried out with a Oxford Supernova CCD diffractometer and a graphite crystal monochromator situated in the incident beam for data collection at 150(2)K.Lorentz polarization and absorption corrections were applied.The structures were solved by direct methods and refined by full-matrix least-squares techniques using the SHELXS-97 and SHELXL-97[29-30]programs.Allnon-hydrogenatomswererefined anisotropically,and hydrogen atoms were located and refined isotropically.Crystallographic data for 1~3 are summarized in Table1.Selected bond lengths and angles were summarized in Table2.
CCDC:1023688,1;776320,2;1023689,3.
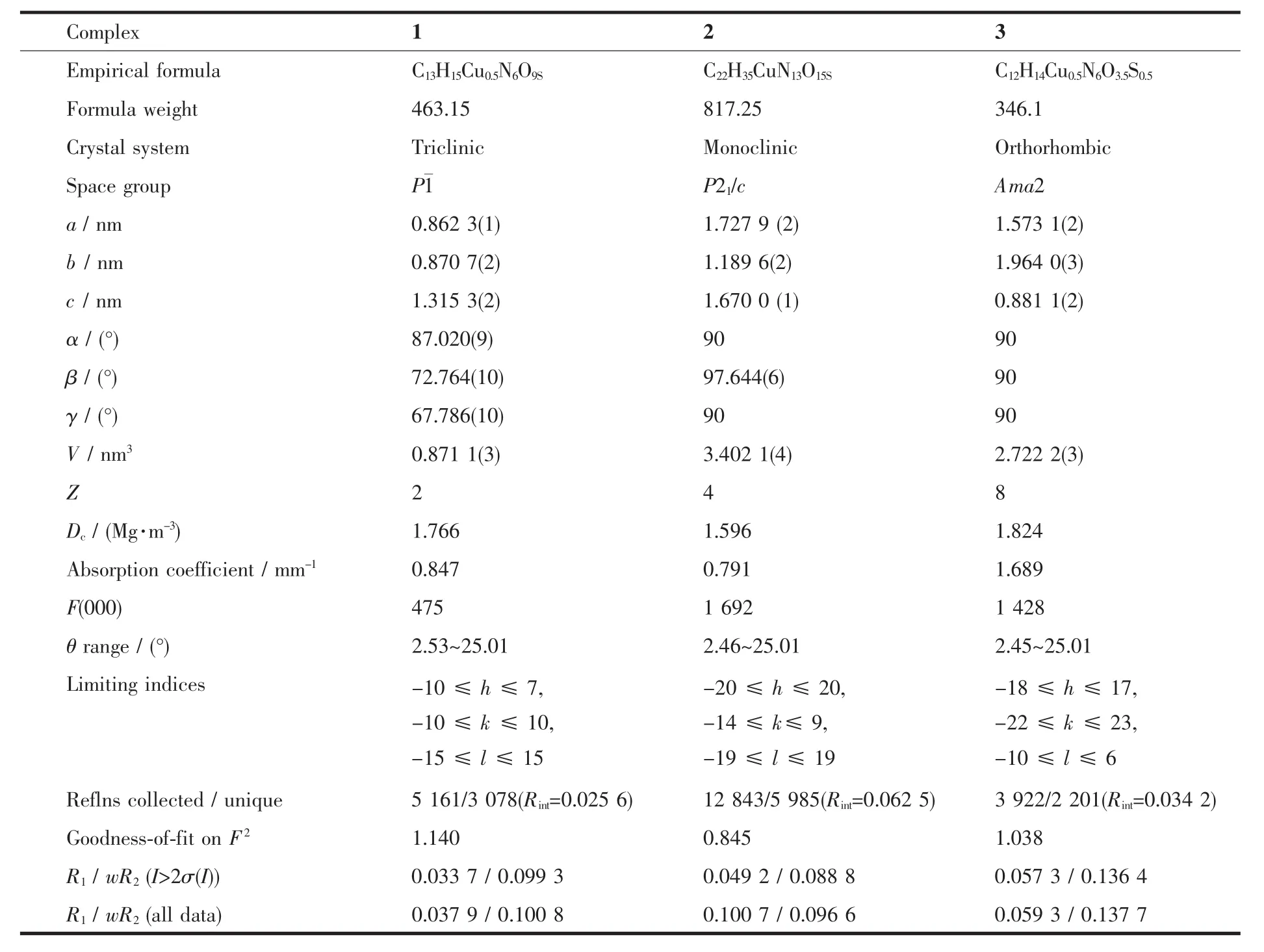
Table1 Crystallographic data and structure refinement for complexes 1~3
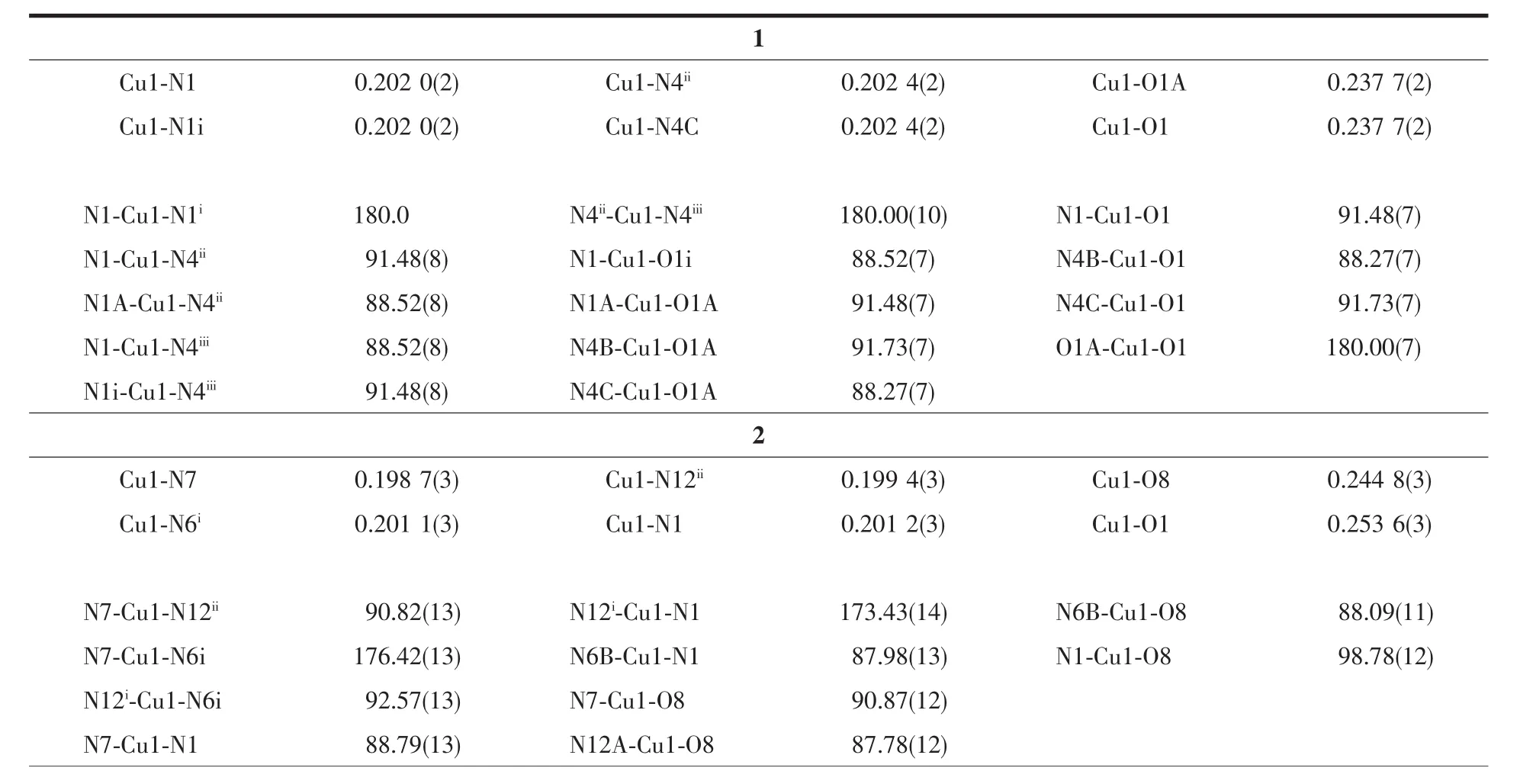
Table2 Selected bond lengths(nm)and bond angles(°)for complexes 1~3
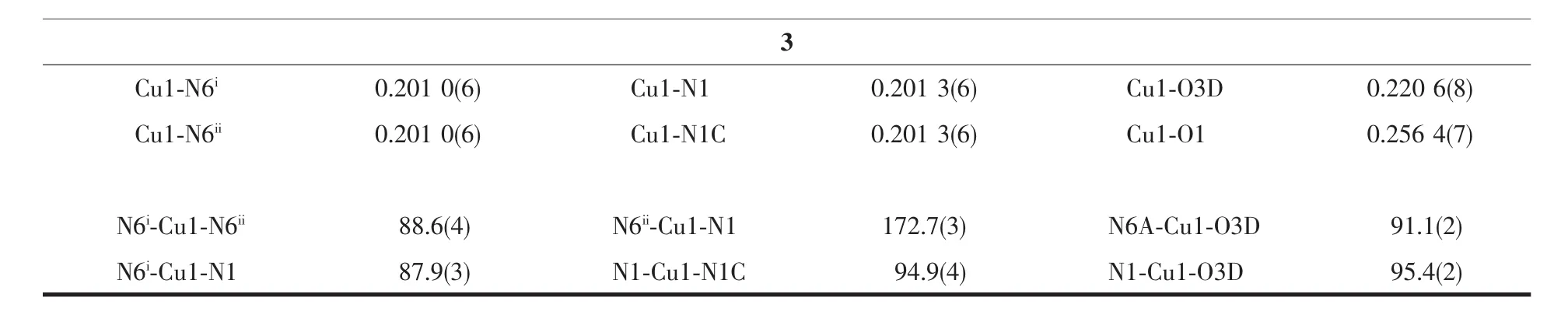
Continued Table2
2 Results and discussion
2.1Description of crystal structure of {[Cu0.5(btm)(H2O)](H2sip)·H2O}n(1)
1 is double-stranded chains which are composed of ribbons of 16-membered rings,each ring involving two copper atoms and two btm molecules,in which the Cu(1)atom is in a general position(Fig.1).The metal coordination sphere is octahedral,with four nitrogen atoms from four btm ligands and two oxygen atoms from two water molecules.The four nitrogen atoms make up the equatorial plane,whereas the two oxygen atoms occupy the apical positions.The Cu-N bonds in 1 are in the normal range,and the axial Cu1-O1 distance(0.237 7(5)nm)is a little longer thanthenormalCu-Odistances,whichcanbe attributed to Jahn-Teller elongation.

Fig.1 (a)Molecule structure of 1,showing the coordination environments of Cu2+,btm and H2sip-ligands;(b)1D chain of 1;Symmetry Codes:ivx,y+1,z;vx,y-1,z; (c)Extended 1D supramolecular double-chain linked with hydrogen-bonding interactions in 1; (d)3D supramolecularstructure of1 viewed from b direction(dashed line:hydrogen bonding)
The btm ligand exhibits cis conformation and works as shorter spacers(N…N 0.581 4 nm).Acting as bidentate chelating-bridging ligands,a pair of btm ligands chelate the Cu1 center by triazolyl N donors with the Cu…Cu separation being 0.870 7(4)nm, which leads to an infinite 1D chain(Fig.1b).
In the framework of 1,free H2sip and lattice water moleculars constitute 1D supramolecular double -chainsalongthecrystallographica-axisthrough classical hydrogen-bonding interactions(O2-H2A…O9vi,O9-H9A…O8ii,O9-H9B…O4viii;Symmetry code:vi-x+2,-y,-z;iix,y-1,z;viii-x+1,-y+1,-z; Table3),which further links the 1D btm-Cu chains into 3D supramolecular architecture(Fig.1c and 1d). The btm-Cu chains carry positive charges,whereas the H2sip--H2O supramolecular chains have negative charge. Thereupon,the3Dsupramolecularstructureisconsolidated by interchain hydrogen bonding interactions as well as electrostatic interactions(Fig.1d).
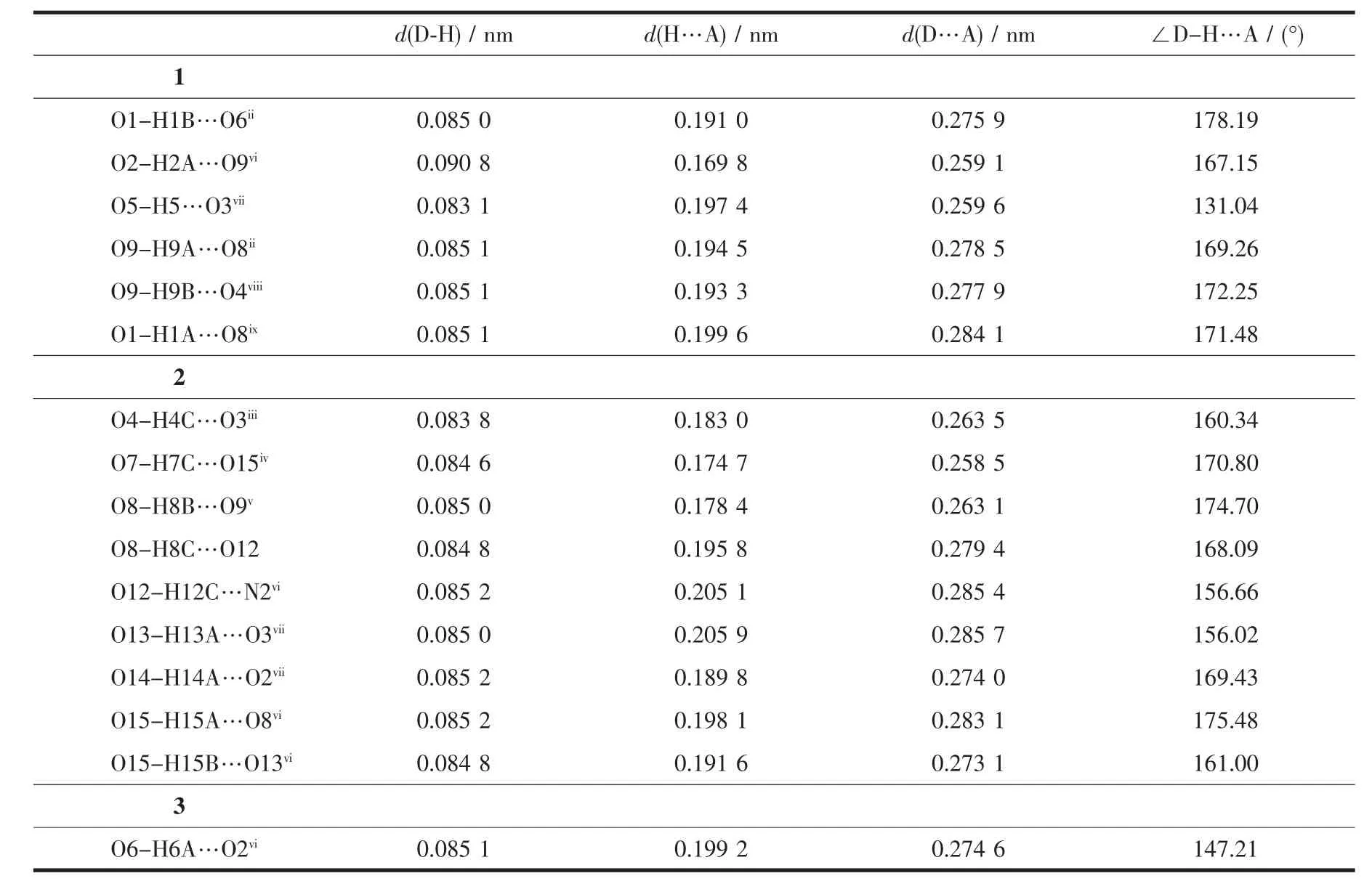
Table3 Selected hydrogen bond data for 1~3
2.2Description of crystal structure {[Cu(btp)2(H2sip)(H2O)](NO3)·4H2O}n(2)
Compound 2 crystallizes in the P21/c space group, and structural determination reveals it as 2D layers linked by μ2-btp ligands.The repeated unit in 2 consists of one crystallographically independent Cu2+ion.As viewed in Fig.2a,Cu1 is six-coordinated in a distortedoctahedralcoordinationspherethatis defined by two different oxygen atoms(one from water, the other two from H2sip-)occupying the axial positions,while the equatorial positions are finished by four nitrogen donors from four btp ligands.The bond distances of Cu1-O8 and Cu1-O1 which occupy the axial positions of the octahedron,are 0.253 6(3)nm and 0.244 5(2)nm,respectively.This axial elongation could be attributed to the Jahn-Teller effect.The other Cu-O and Cu-N bonds in 2 are in the normal range.
All of the btp ligands adopt trans conformation with the N…N distances of 0.743 2 nm(to which N1 belongs)and 0.844 6 nm(to which N7 belongs) between the two donor atoms and a dihedral angle of the two triazole rings of 66.9°and 111.7°,respectively. Each Cuion is linked by four btp ligands,leading to 2D tetragonal layer structures with Cu…Cu separations of 1.002 6 nm(to which N1 belongs)and 1.131 4 nm(to which N7 belongs),as depicted in Fig.2b.The structures provide a very nice example of interdigitation.The sheets occur in which one of the uncoordinated carboxylic groups penetrate the rectangular windows of the partner,as can be clearly seen in Fig. 2c.Whats more,the interdigitating units lead to a 3D supramolecular architecture through the hydrogenbond interactions(O4-H4C…O3iii(iiix,-y+5/2,z-1/2)) (Table3).
2.3Description of crystal structure [Cu(btb)2(Hsip)]n(3)
3 crystallizes in the space group Ama2.In 3,the Cu1 atom and S1 atom lie on a twofold axis and the twofold axis is parallel to the b axis.What′s more,the H2sip-ligand is coordinated to the Cu1 with O1 fromthe-SO3group and O3 from COO-group,so the central atom Cu1 is unsymmetric(Fig.3).That is the reason why compound 3 belongs to non-centrosymmetric space group of Ama2.
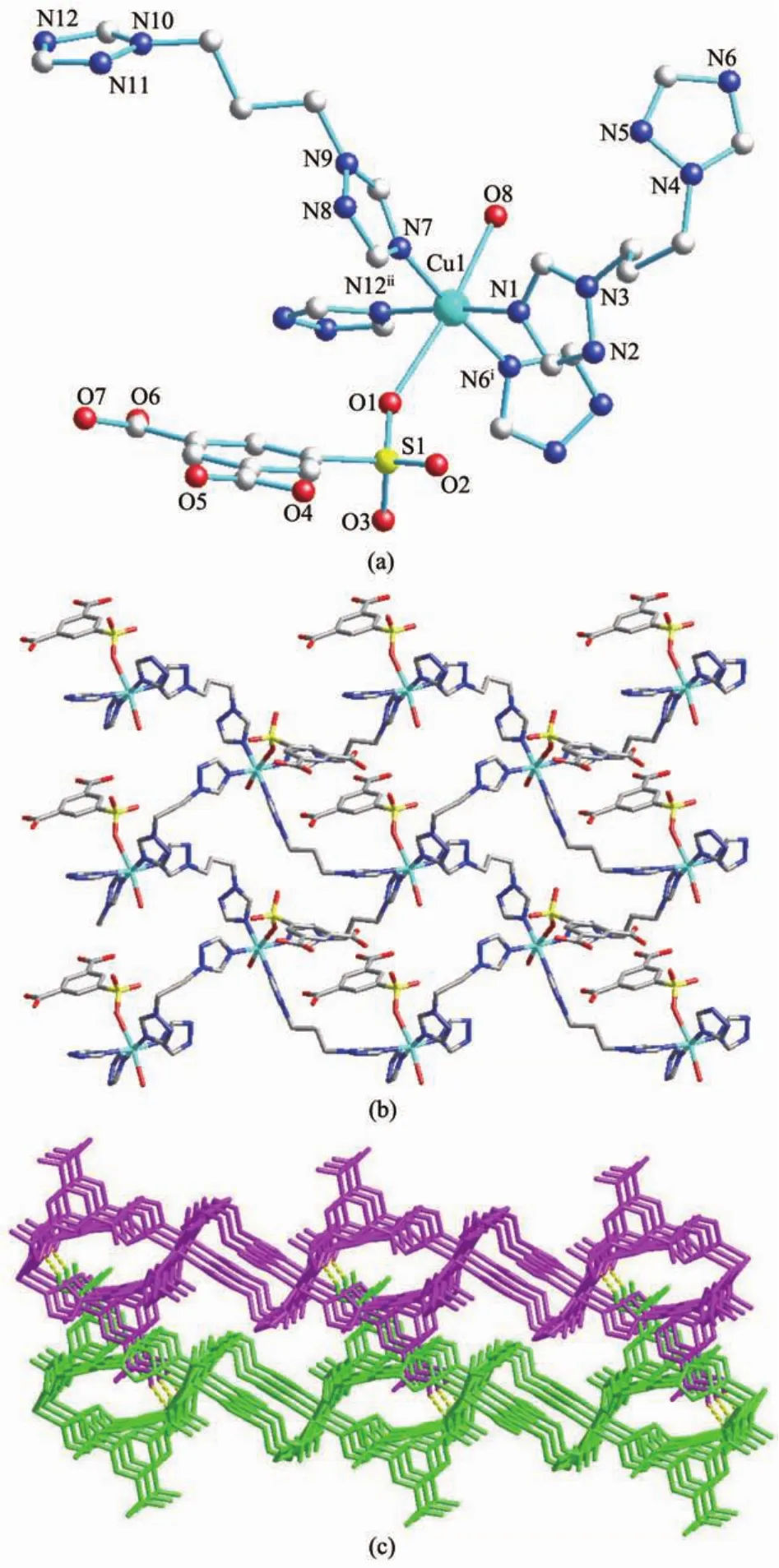
Fig.2 (a)Molecule structure of 2,showing the coordination environments of Cu2+,H2sip-and btp ligands;(b)2D layer of compound 2 in ab plane;(c)3D supramolecular structure of 2 viewed from a direction
The coordination environment of Cuions is presented in Fig.3(a).Every central Cuion is sixcoordinated by two oxygen atoms from two Hsipligands,four nitrogen atoms from four btb ligands, which is in a highly distorted octahedral coordination sphere.The bond distances of Cuions with two Hsip2-oxygen atomsare 0.220 6(8)nm and 0.256 4(7) nm,respectively.This axial elongation could also beattributed to the Jahn-Teller distortion of copperions.All of the other Cu-O and Cu-N bonds are in the normal range.
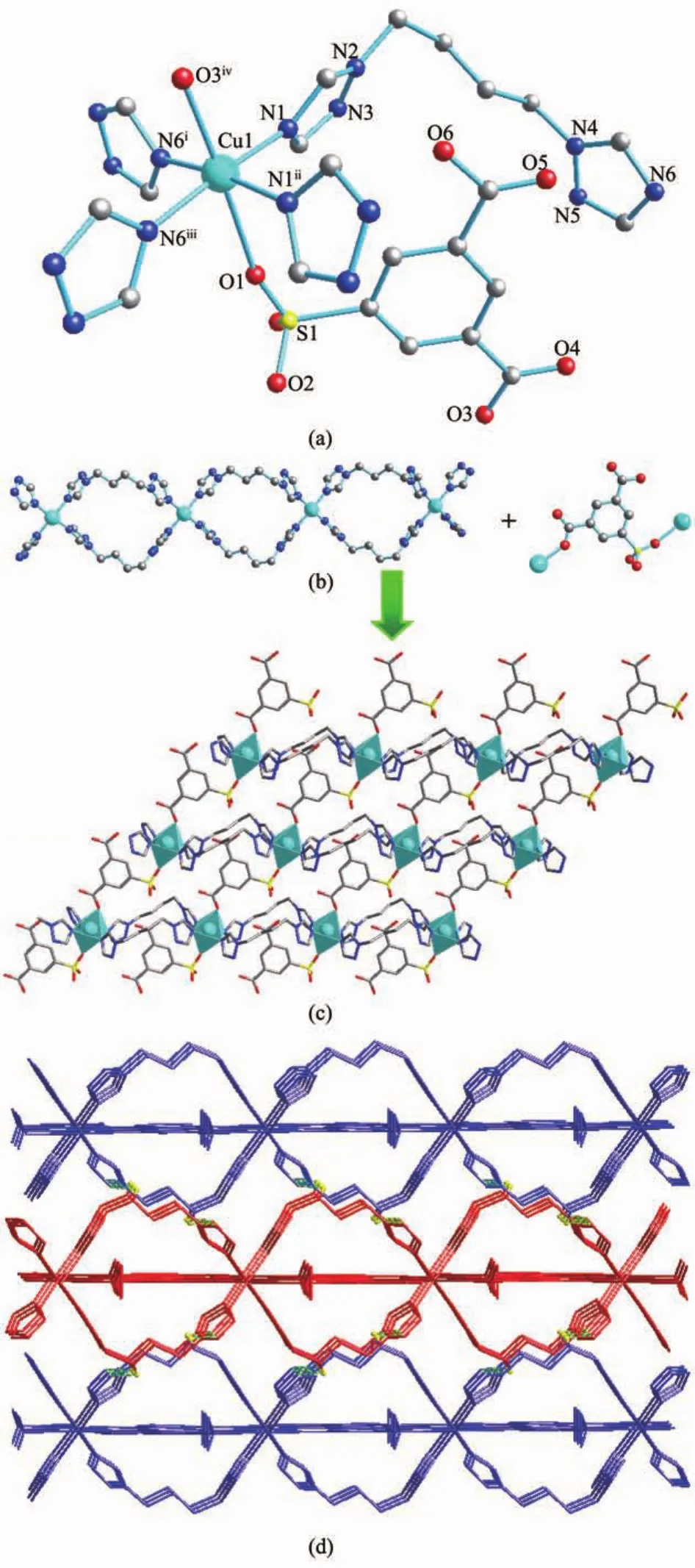
Fig.3 (a)Molecule structure of 3,showing the coordination environments of Cu2+,Hsip2-and btb ligands and building unit with 30%thermal ellipsoids;(b)1D double-stranded chain and μ2-Hsip2-ligand in 3; (c)2D layer of 3 viewed from a direction;(d)3D supramolecular structure of 3 viewed from c direction(dashed line:hydrogen bonding)
The btb ligands adopt cis conformation with the N…N distances of 0.817 1 nm between the two donor atoms and a dihedral angle of the two triazole rings of 83°.Two strands of btb ligands are wrapped around each other and are held together by Cuions,also forming double-stranded chains like compound 1(Fig. 3b).But the Cu…Cu separation across the bridging btb ligand is 1.076 3 nm which is longer than that in 1.In the bc plane,the double-stranded chains are connected into a 2D layer by the link of the μ2-Hsip2-ligands.What′s more,the 2D layers lead to a 3D supramolecular architecture through the weak hydrogen -bond interactions(C12…N5v0.330 4 nm,C12-H12B…N5v126.98°,Symmetry code:v1-x,-y+3/2,z-1/2)).
2.4Comparison of structures
In the construction of compounds 1~3,the difference coordination modes(Scheme 1)of the ligand NaH2sip have an important influence on the resulting supramolecular architectures.In 1,the H2sip-ligand did not coordinated to the central Cu2+,it is only as counter ion.In compound 2,H2sip-ion acts as monodentate ligand,and mode a is observed.In 3,Hsip2-ion coordinated to Cu2+ion in mode b as a bidentate ligand.In addition,the versatile conformations of bistriazole alkanes have an important influence on the resulting frameworks.So compound 1 is doublestranded chains;compounds 2 and 3 are two different kinds of 2D frameworks.This work can be compared with our previous results[19,31-34],in which we used flexible bistriazole and rigid multicarboxylic ligands to obtain 1D,2D,3D and interpenetrated complexes.In allthesecomplexes,althoughbistriazoleligands adopted the same μ2linking mode,ligand conformations,deprotonating degree and coordination modes of the aromatic multi-carboxylic ligands are important for various framework structures in crystal engineering.
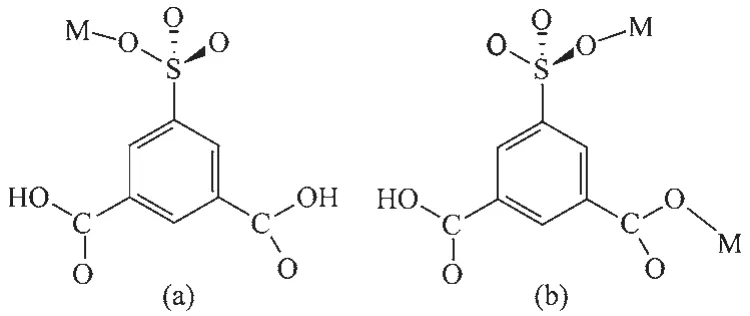
Scheme 1 Coordination modes of ligand NaH2sip in 2 and 3
2.5TGA and EPR characterization for 1~3
The thermal behaviors of these new crystalline materials were studied by thermogravimetric analysis (TGA)under nitrogen atmosphere(Fig.4).Because of the existence of free H2sip anion in 1,it is very unstable and decomposes gradually above 115℃.The TGA result of 2 displays two steps weight losses.For 2,the first weight loss of 11.00%from 105 to 135℃should be attributed to the loss of water molecules (Calcd.11.01%),and the second weight loss is ascribed to the loss of organic ligands and NO3-anions.The decomposition of the coordination framework of 2 occurs immediately when the temperature is above 235℃.The TG curve for 3 reveals that it is stable up to 210℃.With further heating,rapid mass loss occurs,which is assigned to the decomposition of organic ligands.
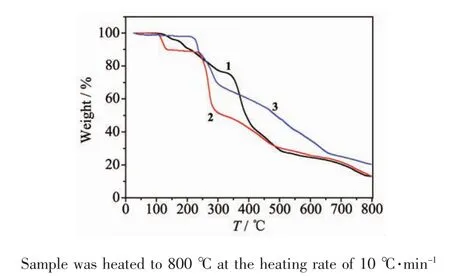
Fig.4 TGA curves for 1~3
The EPR spectra of powdered samples of 1~3 have been measured at the room temperature and are shown in Fig.5.The simulations were carried out by the EasySpin software.The obtained spectra are characteristic for the coppercenters,which are simulated assuming the axial symmetry of g and A tensors.The simulated spectra were obtained by employing the following parameters:g∥=2.21,g⊥=2.05,A1=50 G,A2= 5 G and A3=5 G for compound 1;g1=2.23,g2=2.05,g3= 2.03,A1=90 G,A2=25 G and A3=10 G for compound 2; g1=2.24,g2=2.08,g3=2.05,A1=120 G,A2=0 G and A3= 10 G for compound 3.Both 2 and 3 have three g values, so the Cuions in them exist as unsymmetricallyoctahedral structures.Unlike 2 and 3,the coordination environment of Cuions in 1 appear as symmetrically octahedron,and they only have two g values.The obtained values(g∥>g⊥>2.002 3)for 1 indicate that the unpaired electron is located in the dx2-y2ground state, which is in agreement with the crystal structure and the square-pyramidal coordination around Cu atom.
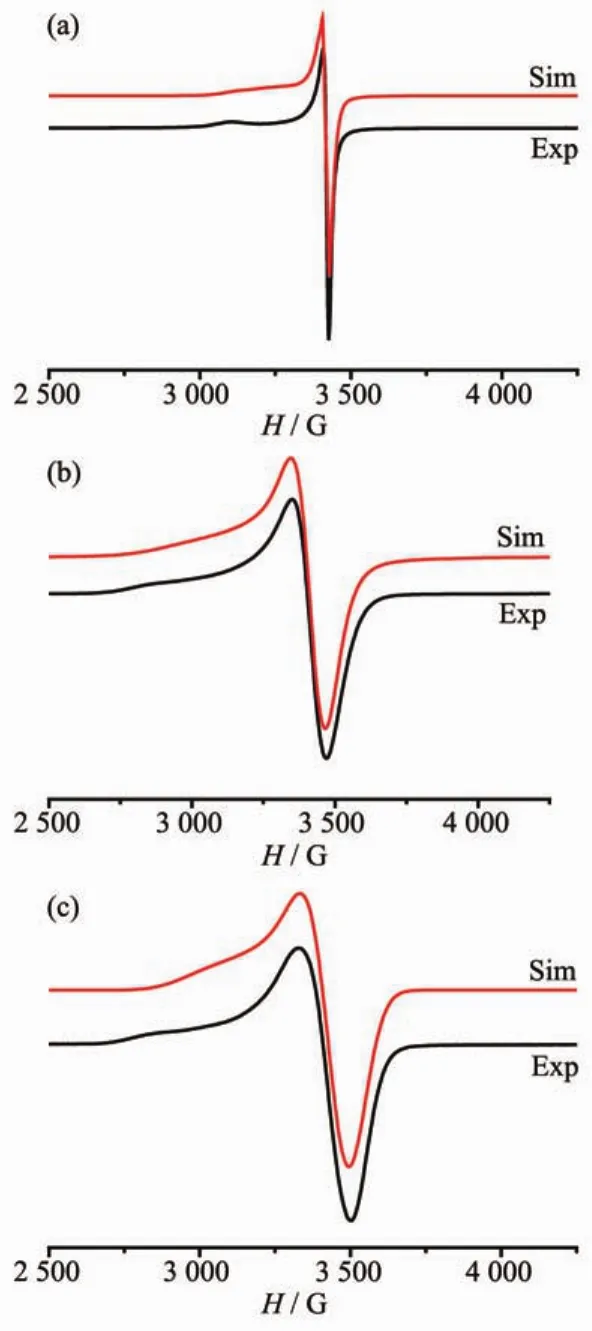
Fig.5 Experimental and simulated X-band EPR spectra of a powdered sample of 1(a),2(b),and 3(c)at room temperature
3 Conclusions
In summary,three novel inorganic-organic frameworks have been constructed from aromatic polycarboxylate acid(NaH2sip)and Cu(NO3)2in the presence of btm,btp and btb ligands with an increase of the length of-(CH2)-.1 features as 1D double-stranded chains.What′s more,the free H2sip-and water moleculars in 1 constitute into a supremolecular 1D chain through classical hydrogen-bonding interactions(O-H…O).2 has two-dimensional(2D)rectangular networks with a(4,4)topology,which contain 2D planar nano grid networks stacked in a step stacking fashion.In compound 3,the flexible btb ligands and Cu2+ions also gives 1D double-strained chains like that in 1, whiles the double-strained chains constitute to novel 2D layers by the link of μ2-Hsip.Structural analyses indicate that the difference in coordination modes of the aromatic polycarboxylic acids NaH2sip and the versatile conformations of bistriazole alkanes have important influences on the resulting frameworks. Meanwhile,the weak hydrogen-bonding interactions also play important roles in the formation of complexes.
Acknowledgments:This work was financially supported by the National Natural Science Foundation of China(No. 21371133),and the Natural Science Fund of Tianjin,China (No.12JCZDJC27600).
[1]Ishikava N,Sugita M,Ishikawa T,et al.J.Am.Chem.Soc., 2003,125:8694-8695
[2]Zhang J P,Lin Y Y,Zhang W X,et al.J.Am.Chem Soc., 2005,127:14162-14163
[3]Biradha K,Sarkar M,Rajput L.Chem.Commun.,2006,11: 4169-4179
[4]Wu C D,Lin W B.Angew.Chem.Int.Ed.,2007,46:1075-1078
[5]Wu S T,Wu Y R,Kang Q Q,et al.Angew.Chem.Int.Ed., 2007,46:8475-8479
[6]Zhang J,Chen S M,Valle H,et al.J.Am.Chem.Soc.,2007, 129:14168-14169
[7]Liu Y,Xu X,Zheng F K,et al.Angew.Chem.Int.Ed., 2008,47:4538-4541
[8]Morris W,Doonan C J,Furukawa H,et al.J.Am.Chem. Soc.,2008,130:12626-12627
[9]Ockwig N W,Delgado-Friedrichs O,OKeeffe M,et al.Acc. Chem.Res.,2005,38:176-182
[10]Biradha K,Sarkar M,Rajput L.Chem.Commun.,2006,11: 4169-4179
[11]Ye B H,Tong M L,Chen X M.Coord.Chem.Rev.,2005, 249:545-565
[12]Hu S,Chen J C,Tong M L,et al.Angew.Chem.Int.Ed., 2005,44:5471-5475
[13]Sun D F,Collins D J,Ke Y X,et al.Chem.Eur.J.,2006, 12:3768-3776
[14]Huang Y G,Yuan D Q,Pan L,et al.Inorg.Chem.,2007,46:9609-9615
[15]Hesham A H,Joaquin S,Christoph J.Dalton Trans.,2008, 37:1734-1744
[16]Habib H A,Hoffmann A,Hppe H A,et al.Dalton Trans., 2009,21:1742-1751
[17]Tian A X,Ying J,Peng J,et al.Cryst.Growth Des.,2008,8: 3717-3724
[18]Chen Z F,Zhang S F,Luo H S,et al.CrystEngComm,2007, 9:27
[19]Tian L,Zhang Z J,Yu A,et al.Cryst.Growth Des.,2010, 10:3847-3849
[20]Ma L F,Han M L,Qin J H,et al.Inorg.Chem.,2012,51: 9431-9442
[21]Ma L F,Zhao J W,Han M L,et al.Dalton Trans.,2012,41: 2078-2083
[22]Sun D F,Cao R,Sun Y Q,et al.Inorg.Chem.,2003,42: 7512-7518
[23]Li B,Zhu X,Zhou J,et al.Polyhedron,2004,23:3133-3141 [24]Wang X,Li B,Zhu X,et al.Eur.J.Inorg.Chem.,2005: 3277-3286
[25]Wu C D,Lin W B.Angew.Chem.Int.Ed.,2005,44:1958-1961
[26]Yi L,Yang X,Lu T B,et al.Cryst.Growth Des.,2005,5: 1215-1219
[27]Zhu X,Ge H,Zhang Y,et al.Polyhedron,2006,25:1875-1883
[28]Torres J,Lavandera J L,Cabildo P,et al.J.Heterocycl. Chem.,1988,25:771-782
[29]Sheldrick G M.SHELXS 97,Program for Crystal Structure Solution,University of G?ttingen,Germany,1997.
[30]Sheldrick G M.SHELXL 97,Program for Crystal Structure Refinement,University of G?ttingen,Germany,1997.
[31]Tian L,Niu Z,Yang N.Inorg.Chim.Acta,2011,370:230-235
[32]Tian L,Yang N,Zhao G Y.Inorg.Chem.Commun.,2010,13: 1497-1500
[33]Tian L,Chen Z.Inorg.Chem.Commun.,2011,14:1302-1305
[34]Tian L,Zhou S Y.J.Coord.Chem.,2013,66:2863-2874
LI TingLI XinZHOU Shang-YongTIAN Li*
(Tianjin Key Laboratory of Structure and Performance for Functional Molecules,Key Laboratory of Inorganic-Organic Hybrid Functional Material Chemistry,Ministry of Education,College of Chemistry,Tianjin Normal University,Tianjin 300387,China)
The reaction of coppernitrate with flexible bis(1,2,4-triazol-1-yl)alkanes and rigid ligand 5-sulfoisophthalic acid monosodium salt(NaH2sip)affords three complexes[Cu0.5(btm)(H2O)](H2sip)·H2O}n(1,btm= bis(1,2,4-triazol-1-yl)methane),{[Cu(btp)2(H2sip)(H2O)](NO3)·4H2O}n(2,btp=1,3-bis(1,2,4-triazol-1-yl)propane),and {[Cu(btb)2(Hsip)]n(3,btb=1,4-bis(1,2,4-triazol-1-yl)butane).Compound 1 contains one-dimensional(1D)doublestrained chains.Compound 2 contains two-dimensional(2D)rectangular networks with(4,4)topology,in which the 2D planar nanogrid networks stacked in a step stacking fashion.3 is also 2D layers,in which double-strained chains[Cu(btb)]nare connected into 2D layer architectures by the μ2-Hsip2-linkers.The three compounds also are characterized by Elemental analysis,EPR,and thermal stability.CCDC:1023688,1;776320,2;1023689,3.
bis(1,2,4-triazol-1-yl)methane;1,3-bis-(1,2,4-triazol-1-yl)propane;1,4-bis(1,2,4-triazol-1-yl)butane; 5-sulfoisophthalic acid monosodium salt;supramolecular structure;hydrogen-bond interaction
O614.121
A
1001-4861(2015)06-1215-09
10.11862/CJIC.2015.145
2015-01-03。收修改稿日期:2015-03-14。
國家自然科學基金(No.21371133)和天津市自然科學基金(No.12JCZDJC27600)資助項目。
*通訊聯系人。E-mail:hxxytl@mail.tjnu.edu.cn,Tel:+86-22-23766515;會員登記號:S06N549M1304。