兩個含有雜環硫酮的銀配合物的合成、晶體結構和光譜特性
崔洋哲 耿文筱 仇啟明 高 森 劉 敏 李中峰 金瓊花*,
(1首都師范大學化學系,北京 100048)(2北京工業大學材料科學與工程學院,北京 100124)
崔洋哲1耿文筱1仇啟明1高森1劉敏2李中峰1金瓊花*,1
(1首都師范大學化學系,北京100048)
(2北京工業大學材料科學與工程學院,北京100124)
合成了2個含有雜環硫酮的銀配合物,[AgBr(PPh3)2(BTZT)]2(1),[Ag2Cl2(PPh3)2(BTZT)2]CH3OH(2)(PPh3=三苯基膦;BTZT=苯并噻唑-2-硫酮),并通過紅外光譜、X射線單晶衍射、熒光光譜和核磁氫譜進行表征。2個配合物是在含有2-巰基苯并噻唑(MBT)的甲醇與二氯甲烷混合溶液中,AgX(X=Cl,Br)與三苯基膦反應得到的。MBT配體由于存在化學活性基團(-N(H)-C(=S)-),所以可以轉化為BTZT配體。結構分析顯示有2個相同的分子結構存在于配合物1中,但是它們具有不同的鍵長和鍵角。配合物2是一個含有菱形的[Ag2Cl2]單元在中心的反轉對稱二聚體,2個相鄰[AgCl(PPh3)(BTZT)]單元由2個氯原子橋連。
熒光;銀;三苯基膦;2-巰基苯并噻唑;苯并噻唑-2-硫酮
0 Introduction
In recent years,an increasing interest has been focused on the development of the silvercomplexes because of their novel crystal structures[1-2]and potential applications as functional materials in catalysis[3-4], luminescence[5-6]and biochemistry[7].Today,many efforts have been done to get a deeper research on the interrelationship between structures and photoluminescence properties of silvercomplexes.
In previous literature,silvercomplexes based on silver halides and organic P-donor ligands such as [AgNO2(PPh3)2][8]and[Ag(PPh3)2(CH3OH)]·BF4[9]are reported.Many silvercomplexes containing PPh3ligand have been drawn much attention,because they can act as functional materials[10-11].2-mercaptobenzothiazole(MBT)is a kind of heterocyclic NS ligand reported frequently because it possesses chemically active groups(-N=C(-SH))(Scheme 1).It used not only as bridging ligand,for example,[Ag6(MBT)6][12]and[Cu6(MBT)6][13],but also as chelating ligand such as[Cd2(MBT)4]n[14].
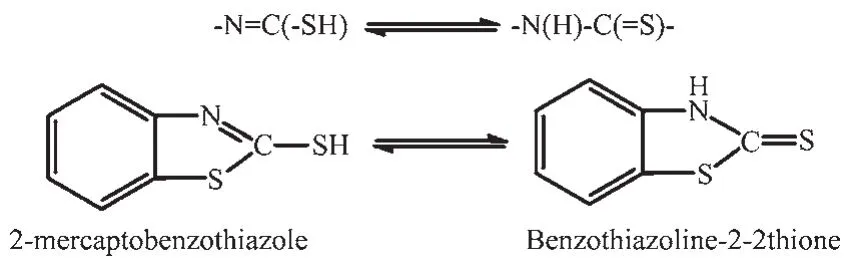
Scheme 1 Equilibrium between 2-mercaptobenzothiazole and benzothiazoline-2-thione
It is reported that different solvents can affect the structures of the products.The solvent CH3OH is the simplest amphiphile which consists of hydrophobic (-CH3)group and hydrophilic(-OH)group.This solvent can not only adjust the weak solubility of the reactant Agsalts effectively but also form hydrogen bonds with the silverhalide.
1 Experimental
1.1Materials and measurements
All chemical reagents are commercially available and used without furthermore treatment.FT-IR spectra (KBr pellets)were measured on a Perkin-Elmer Infrared spectrometer.C,H and N elemental analysis were carried out on an Elementar Vario MICRO CUBE (Germany)elementalanalyzer.Room-temperature fluorescence spectra were measured on F-4500 FL Spectrophotometer.1H NMR was recorded at room temperature with a Bruker DPX 600 spectrometer.
1.2Synthesis of[AgBr(PPh3)2(BTZT)]2
Complex 1 was prepared by reaction of 2-mercaptobenzothiazole(0.033 3 g,0.2 mmol),triphenylphosphine(0.104 7 g,0.4 mmol)and AgBr(0.037 2 g,0.2 mmol)in a mixture of CH3OH and CH2Cl2(10 mL,1∶1,V/V).The solution was stirred for 6 h at room temperature.After slow evaporation of the filtrate at ambient temperature for 6 days,colorless and transparent crystals of the title complex were obtained.Yield: 69%.Element analysis Calcd.(%)for C43H35AgBr NP2S2:C,58.67;H,3.98;N,1.59.Found(%):C, 58.57;H,4.16;N,1.33.IR data(cm-1,KBr pellets): 3 431w,3 053w,2 933w,2 735w,1 596w,1 492m, 1 478m,1 459w,1 431s,1 323m,1 251w,1 180w,1 093 m,1 031m,743s,694s,664m,606w,513m,422w.1HNMR(600 MHz,CDCl3,298 K):δ=13.76(s,NH), 7.52~7.43(m,CHbenzene).
1.3Synthesis of[Ag2Cl2(PPh3)2(BTZT)2]·CH3OH
Complex 2 was prepared in a manner similar to that described for 1,using 2-mercaptobenzothiazole (0.033 5 g,0.2 mmol),triphenylphosphine(0.051 1 g, 0.2 mmol)and AgCl(0.029 3 g,0.2 mmol).Yield:48%. Element analysis Calcd.(%)for C51H44Ag2Cl2N2OP2S4: C,51.97;H,3.74;N,2.38;Found(%):C,51.81;H, 3.71;N,2.38.IR data(cm-1,KBr pellets):3 425w, 3050w,3000w,2 737w,1 598w,1 584w,1 496m,1 478 m,1 458w,1 433s,1 328m,1 253w,1 093m,1 029m, 744s,694s,605w,512s,422w.1H NMR(600 MHz, CDCl3,298 K):δ=7.52~7.42(m,CHbenzene),3.47(s,CH3),1.24(s,OH).
1.4Structure determination
Singlecrystalsofthetitlecomplexeswere mounted on a Bruker Smart 1000 CCD diffractometer equipped with a graphite-monochromated Mo Kα(λ= 0.071 073 nm)radiation.Semi-empirical absorption corrections were applied using SABABS program.All the structures were solved by direct methods using SHELXS program of the SHELXTL-97 package and refined with SHELXL-97[15].Metal atom centers were located from the E-maps and other non-hydrogen atoms were located in successive difference Fourier syntheses.The final refinements were performed by full matrix least-squares methods with anisotropic thermal parameters for non-hydrogen atoms on F2.The hydrogen atoms were generated geometrically and refined with displacement parameters riding on the concerned atoms.
Crystallographic data and experimental details for structural analysis are summarized in Table1,and selected bond lengths and angles of complexes 1~2 are summarized in Table2.The hydrogen bonds of complexes 1~2 are observed in Table3.
CCDC:1035930,1;1043199,2.

Table1 Crystallographic data for complexes 1 and 2

Table2 Selected bond distances(nm)and bond angles(°)for complexes 1 and 2
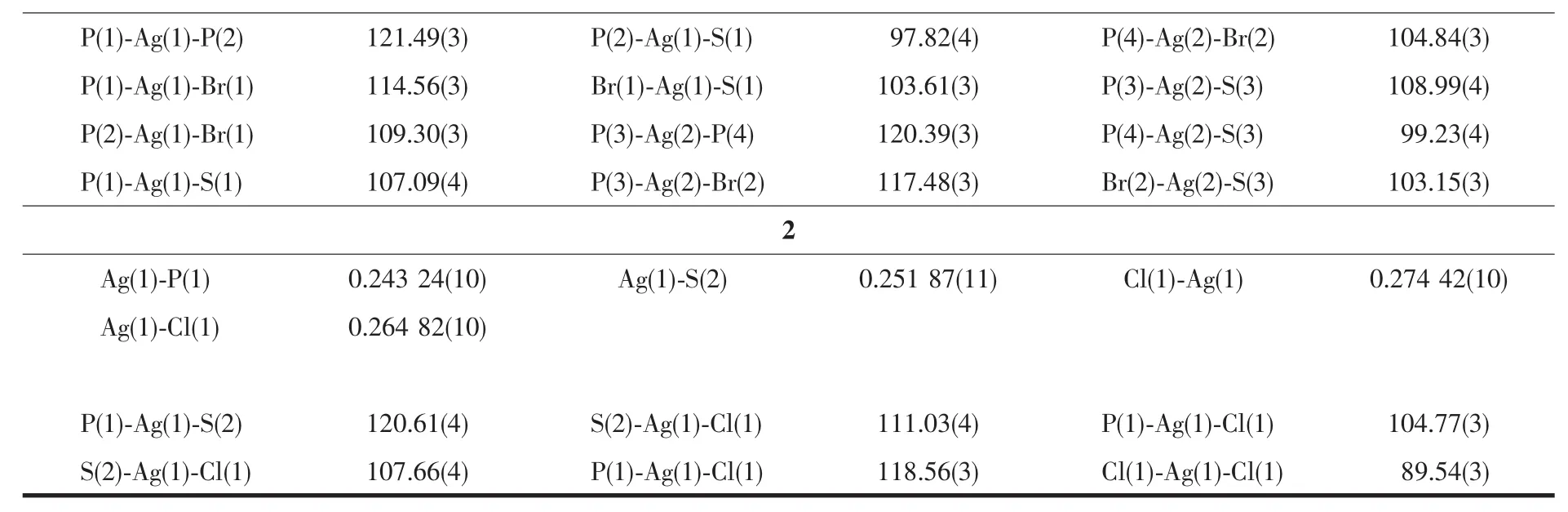
Continued Table2

Table3 Hydrogen bond distances(nm)and bond angles(°)for complexes 1 and 2
2 Results and discussion
2.1Description of the crystal structure
The crystal structure of complex 1 contains two mononuclear[AgBr(PPh3)2(BTZT)]units,but they have different bond lengths and bond angles.Each Ag atom adopts four-coordinated mode,which is coordinated with two P atoms from two PPh3,one Br atom and one S atom from benzothiazoline-2-thione ligand(BTZT) (Fig.1).

Fig.1 Perspective view of complex 1
The coordination pattern of complex 1 is consistent with that of the compound[AgI(PPh3)2(BTZT)][7].The anglesaroundoneAg atom are in the range of 97.82(4)°~121.49(3)°.The angles around the other Ag atom are in the range of 99.23(4)°~120.39(3)°.The coordination geometry around each Ag atom indicates a distorted tetrahedron.Geometrical distortion from ideal angles can be derived from the need to accommodate the bulky PPh3ligand and BTZT ligand.The Ag-Br bond distances(0.269 87(6)nm and 0.270 84(6)nm)are close to those found in:[AgBr(η1-S-H2stsc-NHEt)(PPh3)2][16](0.272 06(3)nm)and[Ag2(μ-Br)2(κ1-S-C3H5NS(N-Et))2(PPh3)2](0.272 47(6)nm)[17].But the Ag-Br bond distances are shorter than that observed in[Ag2(μ-Br)2(κ1-S-C3H5NS(N-Me))2(PPh3)2](0.279 70(5)nm)[17].The Ag-P bond distance is similar with that in previous literature.The Ag-S bond distance in the complex 1 is longer than that observed in[Ag2(μ-S-py-SH)2(PPh3)2Br2] (0.260 8(1)nm)[18].
Moreover,intramolecular N-H…Brhydrogen bonds are observed(N…Br 0.325 6 nm and 0.328 1 nm,N-H…Br 163.95°and 168.81°)in the complex 1.The Br-Ag-S bond angles(103.61(3)°,103.15(3)°) are smaller than the P-Ag-P bond angles(121.49(3)°, 120.39(3)°).The P-Ag-P bond angles(121.49(3)°, 120.39(3)°)in the complex 1 are smaller than that in [Ag(κ1-S-LⅣ-NH)2(PPh3)2](OAc)·H2O(123.846(19)°)(LⅣ-NH=benz-imidazoline-2-thione)[19].
Single-crystal X-ray diffraction analysis reveals that complex 2 consists of inversion symmetric dimers with a diamond-shaped Ag2Cl2group at the center. Each Ag is four-coordinated,surrounded by two Cl atoms,one P atom from one PPh3ligand and one S atom from one BTZT ligand(Fig.2).Two adjacent [AgCl(PPh3)(BTZT)]units are bridged by two Cl atoms to form a dimer.The angles around Agranging from 89.54(3)°to 120.61(4)°indicate that the geometry around Ag atom is distortedly tetrahedral.In the plane,the Ag…Ag distance of 0.382 88(5)nm is longer than the sum of van der Waals radii of two silver atoms(0.344 nm),indicating that there is no metalmetal bonding interaction in the complex 2.The Ag-Cl bond distances(0.264 82(10)nm and 0.274 42(10) nm)are slightly longer than that in the complex[AgCl (η1-S-H2stsc-NHEt)(PPh3)2][16](0.265 91(12)nm).The Ag-S bond distance(0.251 87(11)nm)is close to that found in[Ag(imdt)Cl]n[20](0.248 66(14)nm).The Ag-Cl-Ag bond angle(90.46(3)°)is greater than that in the complex[Ag2(μ-Cl)2(η1-S-Haptsc)2(PPh3)2][21](78.01(2)°). The sum of the internal angles of the four-member ring[-Ag-Cl-Ag-Cl-]in the Ag2Cl2core are 360°, demonstrating that the ring is a parallelogram.
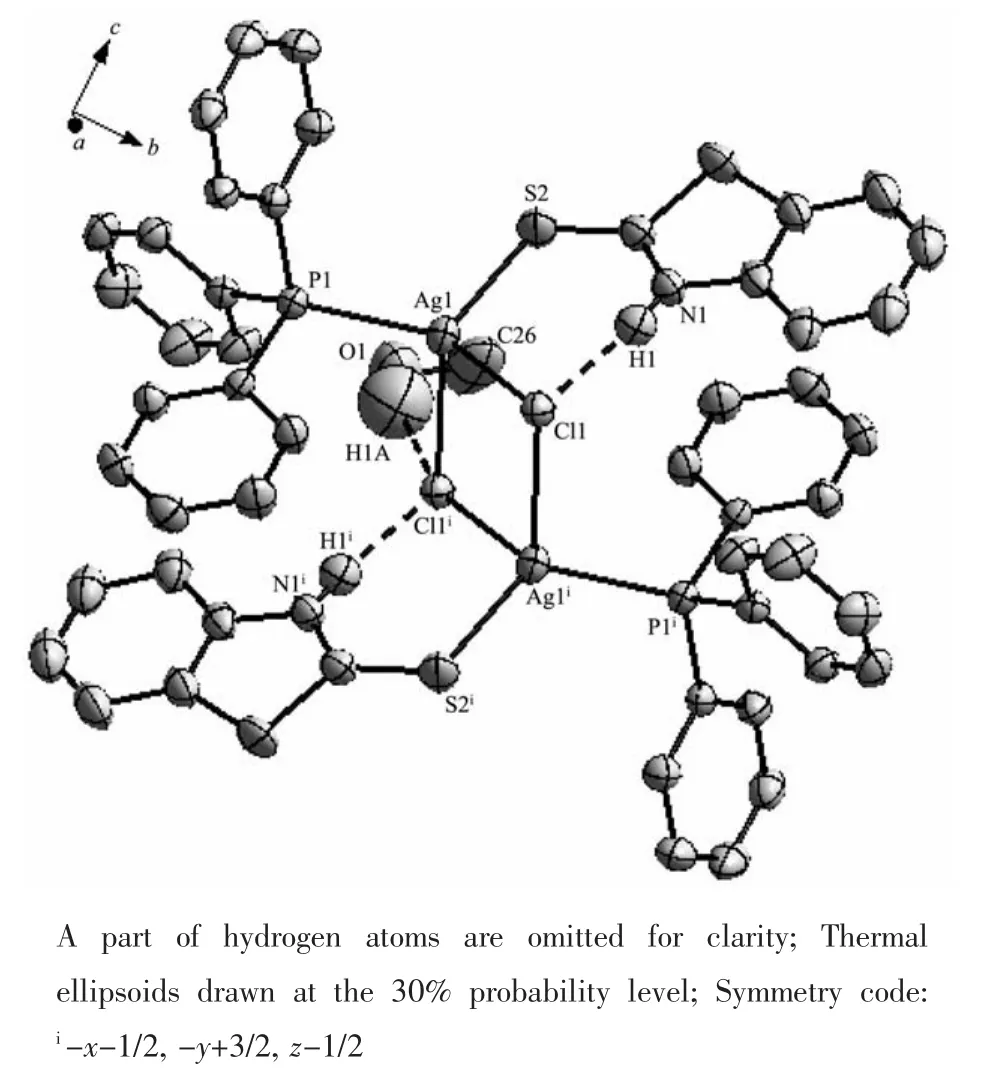
Fig.2 Perspective view of complex 2
Moreover,intramolecular N-H…Cl hydrogen bonds are observed(N…Cl 0.316 1 nm,N-H…Cl 171.11°)in the complex 2,and the hydrogen bond type is consistent with that of complex 1.In addition, the hydrogen bond O1-H1A…Cl1 is formed between O1-H1A group of the free methanol molecule and Cl1 atom of the[AgCl(PPh3)(BTZT)]units.(O…Cl 0.2815 nm,O-H…Cl 165.55°).
2.2Infrared spectroscopy and1H NMR spectroscopy
The infrared spectra of complexes 1 and 2 show the absorption around 1 459~1 492 cm-1due to C-C stretch vibration of the phenyl rings,and the middle absorption around 3 053 cm-1is caused by C-H vibration of the phenyl rings.The C-H out-of-plane bending vibrations of the phenyl rings are found around 744 and694 cm-1.The absorption of the N-H stretch vibration is at 3 431 cm-1.C=N bond vibration is found in 1 431 cm-1and C=S bond vibration is found at 1 251 cm-1.
The1H NMR spectra of complexes 1 and 2 and benzothiazoline-2-thione(BTZT)ligand have been measured at room temperature in CDCl3.The1H NMR spectra of 1 and 2 exist a broad multiple signal between 7.52 and 7.43,which should be the signal of aromatic protons(PPh3or BTZT).Meanwhile in the complex 1,there exists a signal at 13.76,which is attributed to the N-H signal of benzothiazoline-2-thione.In1H NMR spectra of benzothiazoline-2-thione ligand,N-Hsignalofbenzothiazoline-2-thioneis found at 11.44.
2.3Fluorescence spectrum
The solid-state fluorescence emission spectra of the MBT ligand and complexes 1 and 2 are measured at room temperature.The emission and excitation spectra of complexes 1 and 2 is displayed in Fig.3. When excited at 397 nm,complex 1 displays a fluorescence emission peak at 472 nm.When excited at 352 nm,a fluorescence emission peak of complex 2 is found at 424 nm.The emission peak of PPh3is at 402 nm(λex=372 nm)[22].In the fluorescence emission spectra of MBT ligand,the emission peaks are found at 419 nm(λex=342 nm).The red shift of emission peak of 1 and 2 is derived from ligand-centered π-π* transition.

Fig.3 Solid-state excitation and emission spectra of 1 and 2 at 298 K
3 Conclusions
[1]Cui L N,Li Z F,Jin Q H,et al.Inorg.Chem.Commun., 2012,20:126-130
[2]Geng J C,Qin L,Du X,et al.Z.Anorg.Allg.Chem.,2012, 638:1233-1238
[3]Liu H Y,Ji S J,Hao Y H.Z.Anorg.Allg.Chem.,2014,640: 2595-2599
[4]Qin L,Xiao S L,Ma P J,et al.Transition Met.Chem.,2013, 38:627-633
[5]Qiu Q M,Huang X,Zhao Y H,et al.Polyhedron,2014,83: 16-23
[6]Gao S,Li Z F,Liu M,et al.Polyhedron,2014,83:10-15
[7]Banti C N,Kyros L,Geromichalos G D,et al.Eur.J.Med. Chem.,2014,77:388-399
[8]Qiu Q M,Zhao Y H,Liu M,et al.Z.Kristallogr.-New Cryst. Struct.,2014,229:19-20
[9]Gao S,Qiu Q M,Liu M,et al.Z.Kristallogr.-New Cryst. Struct.,2013,228:416-418
[10]Bardaji M,Crespo O,Laguna A,et al.Inorg.Chim.Acta, 2000,304:7-16
[11]Wei Y Q,Wu K C,Zhuang B T,et al.J.Mol.Struct.,2005, 751:133-138.
[12]Chen S C,Yu R M,Zhao Z G,et al.Cryst.Growth Des., 2010,10:1155-1160
[13]Yue C Y,Yan C F,Feng R,et al.Inorg.Chem.,2009,48: 2873-2879
[14]Jin Q H,Zhou L L,Xu L J,et al.J.Chem.Crystallogr., 2010,40:432-436
[15]Sheldrick G M.SHELXS-97 and SHELXL-97,University of G?ttingen,G?ttingen,Germany,1997.
[16]Lobana T S,Kumari P,Kaur I,et al.J.Coord.Chem.,2012, 65:1750-1764
[17]Lobana T S,Sultana R,Butcher R J,et al.J.Organomet. Chem.,2013,745-746:460-469
[18]Lobana T S,Sharma R,Butcher R J.Polyhedron,2008,27: 1375-1380
[19]Lobana T S,Sultana R,Butcher R J,et al.Z.Anorg.Allg. Chem.,2014,640:1688-1695
[20]Zhu Q L,Huang R D,Xu Y Q,et al.J.Coord.Chem.,2009, 62:2656-2664
[21]Lobana T S,Khanna S,Sharma R,et al.Cryst.Growth Des., 2008,8:1203-1212
[22]Huang X,LiZF,QiuQM,etal.Polyhedron,2013,65:129-135
Syntheses,Crystal Structures and Spectroscopic Properties of Two SilverComplexes with Heterocyclic Thione
CUI Yang-Zhe1GENG Wen-Xiao1QIU Qi-Ming1GAO Sen1LIU Min2LI Zhong-Feng1JIN Qiong-Hua*,1
(1Department of Chemistry,Capital Normal University,Beijing 100048,China) (2College of Materials Science and Engineering,Beijing University of Technology,Beijing 100124,China)
Two silvercomplexes with heterocyclic thione,[AgBr(PPh3)2(BTZT)]2(1)and[Ag2Cl2(PPh3)2(BTZT)2]· CH3OH(2)(PPh3=triphenylphosphine;BTZT=benzothiazoline-2-thione)have been synthesized and characterized by IR,single-crystal X-ray diffraction,fluorescence spectrum and1H NMR spectroscopy.1 and 2 are obtained by the reactions of AgX(X=Cl,Br)with PPh3in the presence of 2-mercaptobenzothiazole(MBT)in mixed solvent (CH3OH/CH2Cl2).The MBT ligand possesses chemically active groups(-N=C(-SH)),so MBT ligand can transform into BTZT ligand.Structure analysis shows that there exist two same molecular structures in complex 1,but they have different bond lengths and bond angles.Complex 2 consists of inversion symmetric dimers with a diamondshaped Ag2Cl2group at the center,and two adjacent[AgCl(PPh3)(BTZT)]units are bridged by two Cl atoms. CCDC:1035930,1;1043199,2.
fluorescence;silver;triphenylphosphine;2-mercaptobenzothiazole;benzothiazoline-2-thione
O614.122
A
1001-4861(2015)06-1224-07
10.11862/CJIC.2015.156
2015-01-16。收修改稿日期:2015-03-27。
國家自然科學基金(No.21171119),863國家高技術研究發展計劃(No.2012AA063201),北京教育委員會基金(No.KM201210028020),北京市優秀人才項目(No.2010D005016000002),北京市自然科學基金(No.7122015)資助。
*通訊聯系人。E-mail:jinqh@cnu.edu.cn;會員登記號:S06N3669M1105。