基于2-(4-吡啶基)-1H-咪唑-4,5-二羧酸配體的鎘配合物的晶體結構及發光性質
喬 宇 馬博男 李秀穎 張興晶 尉 兵 侯 婧 車廣波
(1吉林師范大學化學學院,四平 136000)(2環境友好材料制備與應用教育部重點實驗室,四平 136000)
基于2-(4-吡啶基)-1H-咪唑-4,5-二羧酸配體的鎘配合物的晶體結構及發光性質
喬宇1,2馬博男1李秀穎1,2張興晶1,2尉兵1侯婧1車廣波*,2
(1吉林師范大學化學學院,四平136000)
(2環境友好材料制備與應用教育部重點實驗室,四平136000)
在水熱條件下,以2-(4-吡啶基)-1H-咪唑-4,5-二羧酸(H3PIDC)和1,10-菲咯啉衍生物為混合配體合成了2個鎘配合物{[Cd3(HPIDC)3(DPPZ)3]·7H2O}n(1)和[Cd(HPIDC)(Imphen)(H2O)]2(2)(DPPZ=二吡啶并[3,2-a∶2′,3′-c]吩嗪;Imphen=咪唑并[4,5-f] [1,10]菲咯啉),利用元素分析、紅外光譜以及單晶X-射線衍射表征其結構。分析表明配合物1和2分別為一維鏈狀與零維結構。此外,2個配合物展示了優良的熱穩定性及光致發光特性。
鎘配合物;2-(4-吡啶基)-1H-咪唑-4,5-二羧酸;1,10-菲咯啉衍生物;晶體結構;發光
The design and synthesis of metal-organic frameworks(MOFs)are of great interest not only for their potential applications in luminescence[1],absorption[2], catalysis[3]and magnetism[4],but also for their intriguing variety of architectures and fascinating new topologies[5].So far,a large number of mixed-ligand MOFswithversatiledimensionalstructureshavebeen rationally designed and physically characterized[6].The choice of organic ligands is extremely important because they can control and adjust the structures of MOFs.Much attention has been paid to employing the multifunctionalN,O-donorligands,whichusually possess two or more coordination sites and can be assembled around metal ions in various arrangements. These ligands include pyrazine dicarboxylic acids[7], pyridine dicarboxylic acids[8],some carboxyl derivatives of 1,10-phenanthroline[9-10]and imidazole-4,5-dicarboxylic acids ligands.It is well known that imidazole-4,5-dicarboxylic acids are excellent candidates for preparing novel MOFs,because of their versatile coordination modes and potential hydrogen-bonding donors and acceptors.There are some reports of MOFs bearing 2-propyl-imidazole-4,5-dicarboxylic acid[11],2-phenyl-1H-imidazole-4,5-dicarboxylic acid[12],2-(3-methoxyphenyl)-1H-imidazole-4,5-dicarboxylic acid[13], 2-(pyridine-2-yl)-1H-imidazole-4,5-dicarboxylic acid[14], 2-(pyridine-3-yl)-1H-imidazole-4,5-dicarboxylic acid[15], 2-(pyridin-4-yl)-1H-imidazole-4,5-dicarboxylicacid (H3PIDC)[16].Nevertheless,to date,there are no reports concerning MOFs based on imidazole-4,5-dicarboxylic acids and 1,10-phenanthroline′s derivatives mixed ligands.In this context,we report the syntheses and characterization of two novel cadmiumMOFs, {[Cd3(HPIDC)3(DPPZ)3]·7H2O}n(1)and[Cd(HPIDC) (Imphen)(H2O)]2(2)(DPPZ=dipyrido[3,2-a:2′,3′-c] phenazine,Imphen=imidazo[4,5-f][1,10]phenanthroline).Furthermore,the thermal stabilities and luminescence properties of these two complexes have also been investigated.
1 Experimental
1.1Chemicals and general methods
Allchemicalswereusedassuppliedfrom commercialsourceswithoutfurtherpurification. Elemental analyses of carbon,hydrogen and nitrogen were performed on a Perkin-Elmer 240C element analyzer.Fourier transform infrared(FT-IR)spectra was recorded on a Nicolet Nexus 470 FT-IR(America thermo-electricity Company)with 2 cm-1resolution in the range of 4 000~400 cm-1,using KBr pellets. Thermogravimetric analysis(TGA)was performed on a TA Instruments with a heating rate of 10℃·min-1under air atmosphere.The photoluminescent behaviors of the complexes were studied using a Perkin-Elmer LS55 spectrometer.
1.2Syntheses of complexes
1.2.1Synthesis of complex 1
CdSO4·8H2O(0.025 7 g,0.1 mmol),H3PIDC (0.042 2 g,0.2 mmol)and DPPZ(0.056 4 g,0.2 mmol) were dissolved in distilled water(15 mL),and NaOH aqueous solution was added until the pH value of the system was adjusted to about 4.The resulting solution was sealed in a 23 mL Teflon-lined stainless autoclave and heated at 150℃for 3 d under autogenous pressure. After cooling to room temperature,a mixture of yellow block crystals and powder were obtained.The crystals of 1 are picked out from the solid mixture in 43.4% yield based on CdSO4·8H2O.Anal.Calcd.for 1 C84H59N21O19Cd3(%):C,50.35;H,2.97;N,14.68.Found(%): C,50.73;H,3.09;N,14.48.IR spectrum(KBr,cm-1): 3 431(s),1 690(m),1 640(s),1 562(s),1 415(s),1 134 (m),1 021(m),963(w),927(w),809(m),644(s),528(m). 1.2.2Synthesis of complex 2
An identical procedure with 1 was followed to prepare 2 except DPPZ was replaced by Imphen. Yellow crystals of complex 2 suitable for X-ray singlecrystal diffraction analysis were picked out from the solid mixture in 54.8%yield based on CdSO4·8H2O. Anal.Calcd.for 2 C46H30N14O10Cd2(%):C,47.48;H, 2.60;N,16.85.Found(%):C,47.40;H,2.71;N,16.80. IR spectrum(KBr,cm-1):3 424(s),1 686(m),1 577(s), 1 439(s),1 361(s),1 251(m),1 044(m),924(w),804 (m),732(m),649(m),537(w).
1.3Structure determination
Crystallographic data of two complexes were collected at room temperature on a Bruker SMART APEX CCD diffractometer equipped with a graphitemonochromatized Mo Kα(λ=0.071 073 nm)radiation by using an ω-2θ scan method at 292(2)K.All the structuresweresolvedbydirectmethodswith SHELXS-97 program[17]and refined by full-matrix least -squares techniques on F2with SHELXL 97[18].All ofthe non-hydrogen atoms were easily found from the different Fourier map and refined anisotropically, whereas the hydrogen atoms of the complexes were placed by geometrical considerations and were added to the structure factor calculation.In complex 1,the hydrogen atoms attached to water molecules could not be positioned reliably.The detailed crystallographic data and structure refinement parameters for two complexes are summarized in Table1,and selected bond lengths and angles of 1 and 2 are listed in Table2.
CCDC:1040581,1;1040582,2.

Table1 Crystal data and structure refinements for complexes 1 and 2

Table2 Selected bond lengths(nm)and bond angles(°)for complexes 1 and 2
2 Results and discussion
2.1Description of the crystal structures
2.1.1Structure description of 1
As shown in Fig.1,the asymmetric unit of 1 contains one point five crystallographically independent Cd2+cations(Cd1 and Cd2),one point five HPIDC2-ligands,one point five DPPZ ligands and three pointfive lattice water molecules.Cd2 lies in general positions,while Cd1 is located at an inversion center. Cd2 and Cd1 are all six coordinated O2N4and reside in the distorted octahedral coordination environment: two oxygen atoms(O4,O5 or O1,O1i,Symmetry code:i-x+1,-y+1,-z+1)from two different HPIDC2-ligands,and four nitrogen atoms(N1,N2,N6,N10 or N8,N8i,N5,N5i,Symmetry code:i-x+1,-y+1,-z+1) from one DPPZ ligand and two different HPIDC2-ligands.The Cd-O and Cd-N bond lengths are in the range of 0.237 6(8)~0.250 1(11)nm and 0.224 3(9)~0.238 6(9)nm,respectively.All Cd-O and Cd-N bond lengths are in agreement with those reported in other Cd2+coordination compounds[19].

Fig.1 Coordination environments of Cd2+atoms in complex 1 with displacement ellipsoids at 30% probability level
The neighboring two Cd2+units are linked by one HPIDC2-ligand in bis-chelating mode(Scheme 1(a) and 1(b))to form an infinite 1D chain along the a axis with all the DPPZ ligands attached to three sides,as shown in Fig.2.The Cd2…Cd1 and Cd2…Cd2iidistances bridged by the one HPIDC2-ligand are 0.674 1 and 0.671 2 nm,respectively.The neighboring 1D chains interact through π-π stackings between the DPPZ ligands(centroid-to-centroid distance ca.0.343 2 nm,Fig.3)to yield a two-dimensional layer structure.

Scheme 1 Coordination patterns of H3PIDC ligand in complexes 1 and 2

Fig.2 One-dimensional chain structure of complex 1 along a axis

Fig.3 Two-dimensional layer structure of 1
Although the HPIDC2-ligand in complex 1 has onlyonebis-chelating coordination mode,two carboxylic groups in a H3PIDC ligand exist in partially(Scheme 1(a))and fully(Scheme 1(b))deprotonated forms.It is remarkable that there are the partially protonated pyridine of HPIDC2-ligands in complex 1 and it is not surprising to find protonated pyridine[20].
2.1.2Structure description of 2
As shown in Fig.4(a),the crystal structure of 2 consists of a discrete dinuclear[Cd(HPIDC)(Imphen) (H2O)]2.The[Cd(HPIDC)(Imphen)(H2O)]2dimer comprises two HPIDC2-ligands,two Imphen ligands, two water molecules and two Cdatoms.Each Cdatom in 2 is coordinated by three nitrogen atoms(N1 and N2 atoms from a chelating Imphen,and N5 from a HPIDC2-ligand),two oxygen atoms(O3 and O3iatoms from two different HPIDC2-ligands)and one water molecule in a slightly distorted octahedral geometry.Coordination mode of H3PIDC ligand in complex 2 is shown in Scheme 1(c).

Fig.4 (a)Coordination environment of Cd2+in complex 2 with displacement ellipsoids at 30%probability level(All the hydrogen atoms are omitted for clarity;Symmetry code:i-x+1,-y+1,-z+1);(b)One-dimensional chain structure of 2;(c)Two-dimensional layer structure of 2;(d)Three-dimensional supramolecular network of 2 via hydrogen bonds and π-π interactions
The distances of the Cd-O bond(0.225 7(3)~0.2330(3)nm)andCd-Nbond(0.2320(3)and0.2344(3) nm)are near to those of reported[19].The adjacent Cdatoms are bridged by two HPIDC2-ligands to form a dimer with Cd…Cd distance of 0.373 9 nm. The neighboring discrete dimers are assembled via ππ stacking interactions between two HPIDC2-ligands (atom-to-centroid distance:0.352 4 nm)into a 1D chain motif(Fig.4(b)),and then the adjacent chain further interact to form a 2D layer structure through π-π interactions between Imphen ligands(atom-tocentroid distance:0.345 4 nm,Fig.4(c)).Simultaneously, these 2D layers ulteriorly stacked to furnish a 3D supramolecular network(Fig.4(d))via π-π interactions between Imphen ligands(centroid-to-centroid distance ca.0.366 5 nm).In addition,the N-H…N,O-H…N, O-H…O hydrogen bonds(Table3)involving Imphen ligands,HPIDC2-ligands and the water molecules play a vital role in further extend and consolidate the 0D dimers arrays into an interesting 3D supramolecularstructure.

Table 3 Hydrogen bond lengths(nm)and bond angles(°)of complex 2
2.2TGA
Thermogravimetric experiment of complexes 1 and 2 was performed to explore their thermal stabilities. As shown in Fig.5,two distinct weight losses were observed for these two complexes.The first obvious weight loss of 6.01%and 3.20%for 1 and 2 are in the range of 97~140℃and 140~220℃,assigned to the release of the water molecules(Calcd.6.29%and 3.09%,respectively).The second sharp weight loss of 77.29%and 75.99%are ascribable to the decomposition of organic ligands HPIDC2-and DPPZ/Imphen (Calcd.76.83%:HPIDC2-34.61%and DPPZ 42.22% for 1;Calcd.77.58%:HPIDC2-39.73%and Imphen 37.85%for 2)occurring from 313 to 670℃and 285 to 653℃,which means the degradation of the frameworks.The final products may be CdO for complexes 1 and 2.

Fig.5 TG curves of complexes 1 and 2
2.3Photoluminescence properties
The photoluminescent emission properties of 1 and 2 were investigated in the solid state at room temperature.The free ligands H3PIDC,DPPZ and Imphen exhibit the strongest emission peaks at about 470 nm[19],444 nm[21]and 460 nm[22]from 300 to 720 nm,respectively.Compared to the free ligands, the strongest emission peaks for 1 and 2 are at 582 and 545 nm(Fig.6,excitation at 365 nm),respectively. According to the documents,the Cdions is difficult to oxidize or reduce because of the d10configuration. As a result,the emissions of the two complexes are neither metal-to-ligand charge transfer(MLCT)nor ligand-to-metal charge transfer(LMCT).Compared with the luminescent spectra of free ligands,the emission spectra of two complexes are red-shifted and obviously similar to that of H3PIDC,DPPZ or Imphen.So,the photoluminescence of 1 and 2 might originate from the intraligand π→π*transitions mainly through the H3PIDC and/or DPPZ/Imphen ligands,namely ligandto-ligand charge transfer(LLCT)[19,23].The reason of spectra red-shift are considered to mainly arise from the increased rigidity of the organic ligands when coordinating to Cdions.
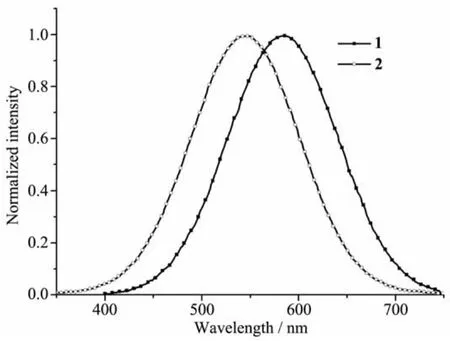
Fig.6 PL spectra of complexes 1 and 2
3 Conclusions
Two Cd2+complexes formulated as{[Cd3(HPIDC)3(DPPZ)3]·7H2O}n(1)and[Cd(HPIDC)(Imphen)(H2O)]2(2)have been hydrothermally synthesized and structurally characterized.The complex 1 exhibits 1D chain structure,and further extends to 2D layer through ππ stacking between the DPPZ ligands.The complex 2 shows a discrete dimer,and these dimers stacks to furnish a 3D supramolecular layer structure via π-π interactions between two ligands.The thermogravimetric analyses data indicate that the framework structures of complexes 1 and 2 are thermally stable up to 285℃.The complexes 1 and 2 exhibit strong fluorescent emissions in the visible region at room temperature.
[1]Zhang S,Yang Y,Xia Z Q,et al.Inorg.Chem.,2014,53: 10952-10963
[2]Hwang I H,Kim H Y,Lee M M,et al.Cryst.Growth Des., 2013,13:4815-4823
[3]Yi X C,Xi F G,Qi Y,et al.RSC Adv.,2015,5:893-900
[4]Chen W X,Tan L,Liu Q P,et al.Dalton Trans.,2014,43: 16515-16521
[5]Fernandez C A,Liu J,Thallapally P K,et al.J.Am.Chem. Soc.,2012,134:9046-9049
[6]Stock N,Biswas S.Chem.Rev.,2012,112:933-969
[7]Masci B,Thuéry P.Cryst.Growth Des.,2008,8:1689-1696
[8]Yang A H,Zou J Y,Wang W M,et al.Inorg.Chem.,2014, 53:7092-710
[9]Liu C B,Wang S S,Che G B,et al.Inorg.Chem.Commun., 2013,27:69-75
[10]Liu C B,Cong Y,Sun H Y,et al.Inorg.Chem.Commun., 2014,47:71-74
[11]Deng J H,Zhong D C,Luo X Z,et al.Cryst.Growth Des., 2012,12:4861-4869
[12]Wang W Y,Niu X L,Gao Y C,et al.Cryst.Growth Des., 2010,10:4050-4059
[13]Xiong Z F,Shi B B,Li L,et al.CrystEngComm,2013,15: 4885-4899
[14]Li X,Wu B L,Zhang H Y,et al.Inorg.Chem.,2010,49: 2600-2613
[15]Yang L Y,Zeng L F,Gu W,et al.Inorg.Chem.Commun., 2013,29:76-81
[16]Sun L,Li G Z,Xu M H,et al.Eur.J.Inorg.Chem.,2012: 1764-1772
[17]Sheldrick G M.SHELXS 97,Program for the Solution of Crystal Structure,University of G?ttingen,1997.
[18]Sheldrick G M.SHELXS 97,Program for the Refinement of Crystal Structure,University of G?ttingen,1997.
[19]Yuan G,Shao K Z,Du D Y,et al.CrystEngComm,2012,14: 1865-1873
[20]Liao Z L,Li G D,Bi M H,et al.Inorg.Chem.,2008,47: 4844-4853
[21]Yang J,Li G D,Cao J J,et al.Chem.Eur.J.,2007,13:3248 -3261
[22]Liu J Q,Zhang Y N,Wang Y Y,et al.Dalton Trans.,2009: 5365-5378
[24]Choppin G R,Peterman D R.Coord.Chem.Rev.,1998,174: 283-299
QIAO Yu1,2MA Bo-Nan1LI Xiu-Ying1,2ZHANG Xing-Jing1,2WEI Bing1HOU Jing1CHE Guang-Bo*,2
(1College of Chemistry,Jilin Normal University,Siping,Jilin 136000,China) (2Key Laboratory of Preparation and Applications of Environmental Friendly Materials, Chinese Ministry of Education,Jilin Normal University,Siping,Jilin 136000,China)
Two cadmiumcomplexes based on 2-(pyridin-4-yl)-1H-imidazole-4,5-dicarboxylic acid(H3PIDC)and 1,10-phenanthroline′s derivatives mixed ligands,namely,{[Cd3(HPIDC)3(DPPZ)3]·7H2O}n(1)and[Cd(HPIDC) (Imphen)(H2O)]2(2)(DPPZ=dipyrido[3,2-a∶2′,3′-c]phenazine,Imphen=imidazo[4,5-f][1,10]phenanthroline),have been synthesized under hydrothermal conditions and characterized by elemental analysis,infrared spectrum and single-crystal X-ray diffraction.Complexes 1 and 2 are one-dimensional chain and zero-dimensional structures, respectively.Two complexes exhibit excellent thermal stabilities and photoluminescent properties.CCDC:1040581, 1;1040582,2.
cadmiumcomplex;2-(pyridin-4-yl)-1H-imidazole-4,5-dicarboxylic acid;1,10-phenanthroline′s derivative; crystal structure;luminescence
O614.24+2
A
1001-4861(2015)06-1245-07
10.11862/CJIC.2015.168
2015-02-22。收修改稿日期:2015-04-07。
教育部新世紀優秀人才(No.NCET-10-0176),吉林省科技廳(No.20130521019JH、201215219)資助項目。
*通訊聯系人。E-mail:guangbochejl@yahoo.com;guangboche@jlnu.edu.cn