孟魯司特片聯合沙美特羅替卡松粉吸入劑治療咳嗽變異性哮喘的臨床療效
王 永,楊雅淳,劉 勇
·藥物與臨床·
孟魯司特片聯合沙美特羅替卡松粉吸入劑治療咳嗽變異性哮喘的臨床療效
王 永,楊雅淳,劉 勇
221400江蘇省新沂市,鐵路醫院呼吸科(王永,劉勇),藥劑科(楊雅淳)
【摘要】目的觀察孟魯司特片聯合沙美特羅替卡松粉吸入劑治療咳嗽變異性哮喘(CVA)的臨床療效。方法選取2011—2014年新沂市鐵路醫院收治的CVA患者112例,按就診順序分為對照組與觀察組,每組56例。對照組患者予以沙丁胺醇氣霧劑治療,觀察組患者予以孟魯司特片聯合沙美特羅替卡松粉吸入劑治療;兩組患者均連續治療8周。比較兩組患者臨床療效、治療前后肺功能指標〔第1秒用力呼氣容積(FEV1)、第1秒用力呼氣容積占用力肺活量的百分比(FEV1/FVC)、最大呼氣流量(PEF)〕、咳嗽緩解時間(CRT),咳嗽消失時間(CDT)、復發率(RR)及不良反應發生情況。結果觀察組患者臨床療效優于對照組(P<0.05)。治療前兩組患者FEV1、FEV1/FVC、PEF比較,差異無統計學意義(P>0.05);治療后觀察組患者FEV1、FEV1/FVC、PEF高于對照組(P<0.05)。觀察組患者CRT、CDT短于對照組,RR低于對照組(P<0.05)。對照組患者出現頭痛3例,手指震顫2例;觀察組患者未出現嚴重不良反應。結論孟魯司特片聯合沙美特羅替卡松粉吸入劑治療CVA的臨床療效確切,可改善患者肺功能,縮短患者咳嗽緩解時間及消失時間,降低復發率,且安全性較高。
【關鍵詞】哮喘;孟魯司特片;沙美特羅替卡松粉吸入劑;治療結果
王永,楊雅淳,劉勇.孟魯司特片聯合沙美特羅替卡松粉吸入劑治療咳嗽變異性哮喘的臨床療效[J].實用心腦肺血管病雜志,2016,24(4):118-120.[www.syxnf.net]
Wang Y,Yang YC,Liu Y.Clinical effect of montelukast combined with salmeterol and fluticas inhalant on cough variant asthma[J].Practical Journal of Cardiac Cerebral Pneumal and Vascular Disease,2016,24(4):118-120.
咳嗽變異性哮喘(cough variant asthma,CVA)是呼吸內科常見病、多發病,臨床表現為慢性或持續性發作的咳嗽,是一種特殊類型哮喘[1],嚴重影響患者的身心健康及生活質量,其臨床癥狀、體征與上呼吸道感染、支氣管炎、咽炎等相似,易造成誤診、漏診[2],其占慢性咳嗽病因的14%~42%[3]。近年來,隨著大氣及環境污染加劇,CVA發病率及復發率呈逐年上升趨勢。CVA常因誤診而導致治療不當,療程不足,治療效果不佳,病情反復,給患者造成較大的壓力,故CVA需早期診斷,并積極進行干預治療。目前,CVA尚無統一的臨床治療方案[4],治療方法和藥物與典型哮喘基本相同[5]。本研究旨在探討孟魯司特片聯合沙美特羅替卡松粉吸入劑治療CVA的臨床療效,現報道如下。
1資料與方法
1.1納入與排除標準納入標準:(1)符合中華醫學會呼吸病學分會哮喘學組制定的CVA診斷標準[6];(2)年齡≥16歲;(3)胸部X線、CT檢查未見異常。排除標準:(1)嚴重心、肝、腎功能障礙者;(2)妊娠期及哺乳期婦女;(3)精神病者;(4)合并呼吸道感染者;(5)合并其他呼吸道疾病者;(6)不能或不愿配合治療者。
1.2一般資料選取2011—2014年新沂市鐵路醫院收治的CVA患者112例,本研究獲得醫院倫理委員會批準,患者及其家屬簽署知情同意書。按就診順序將所有患者分為觀察組與對照組,各56例。兩組患者性別、年齡、病程比較,差異無統計學意義(P>0.05,見表1),具有可比性。
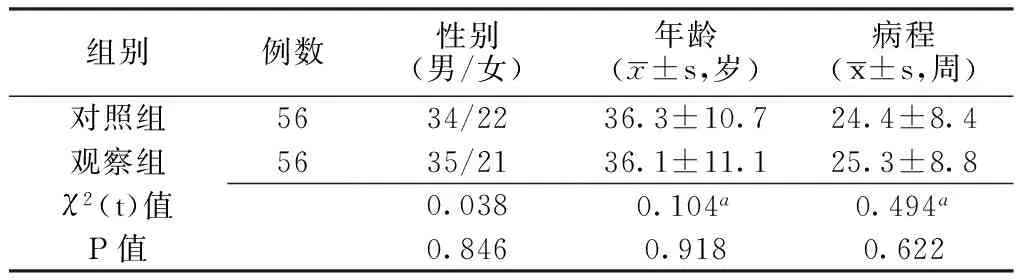
表1 兩組患者一般資料比較
注:a為t值
1.3方法對照組患者予以沙丁胺醇氣霧劑(葛蘭素史克集團公司生產,批準文號:H20090514;規格:100 μg/噴)治療, 1~2噴/次,4次/d。觀察組患者予以孟魯司特片(杭州默沙東制藥有限公司生產,批準文號:J20070070;規格:5 mg/片)聯合沙美特羅替卡松粉吸入劑(葛蘭素史克集團公司生產,批準文號:H20090240;規格:50/250 μg/噴) 治療,孟魯司特片10 mg/次,口服,1次/d;沙美特羅替卡松粉吸入劑1噴/次,2次/d。兩組患者均連續治療8周。
1.4觀察指標觀察兩組患者臨床療效、治療前后肺功能指標〔第1秒用力呼氣容積(FEV1)、第1秒用力呼氣容積占用力肺活量的百分比(FEV1/FVC)、最大呼氣流量(PEF)〕、咳嗽緩解時間(CRT),咳嗽消失時間(CDT)、復發率(RR)及不良反應發生情況。采用日本捷斯特HI-101型肺功能儀檢測肺功能指標;隨訪6個月記錄患者復發情況,計算RR,RR=復發例數/總例數×100%。
1.5臨床療效判定標準治愈:治療后患者咳嗽消失,肺功能恢復正常;顯效:治療后患者咳嗽發作次數及程度明顯好轉,肺功能明顯改善;有效:治療后患者咳嗽發作次數及程度有所好轉,肺功能有所改善;無效:治療后患者咳嗽發作次數及程度無好轉或出現加重,肺功能改善不明顯或未改善。
2結果
2.1臨床療效觀察組患者臨床療效優于對照組,差異有統計學意義(u=2.378,P<0.05,見表2)。

表2 兩組患者臨床療效比較〔n(%)〕
2.2肺功能指標治療前兩組患者FEV1、FEV1/FVC、PEF比較,差異無統計學意義(P>0.05);治療后觀察組患者FEV1、FEV1/FVC、PEF高于對照組,差異有統計學意義(P<0.05,見表3)。
Table3Comparisonoflungfunctionindexbetweenthetwogroupsbeforeandaftertreatment
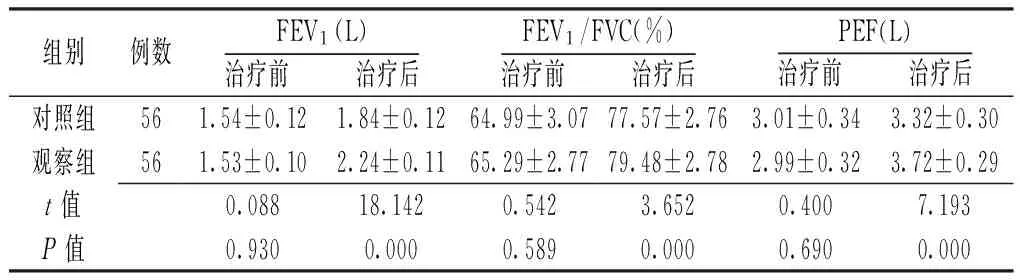
組別例數FEV1(L)治療前 治療后FEV1/FVC(%)治療前 治療后PEF(L)治療前 治療后對照組561.54±0.121.84±0.1264.99±3.0777.57±2.763.01±0.343.32±0.30觀察組561.53±0.102.24±0.1165.29±2.7779.48±2.782.99±0.323.72±0.29t值0.08818.1420.5423.6520.4007.193P值0.9300.0000.5890.0000.6900.000
注:FEV1=第1秒用力呼氣容積,FEV1/FVC=第1秒用力呼氣容積占用力肺活量的百分比,PEF=最大呼氣流量
2.3CRT、CDT、RR觀察組患者CRT、CDT短于對照組,RR低于對照組,差異有統計學意義(P<0.05,見表4)。
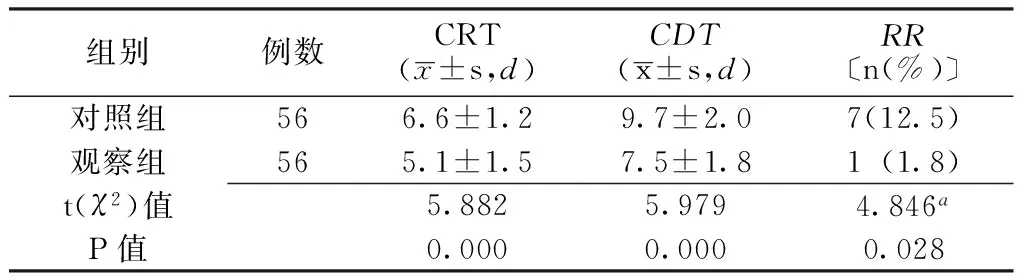
表4 兩組患者CRT、CDT、RR比較
注:CRT=咳嗽緩解時間,CDT=咳嗽消失時間,RR=復發率;a為χ2值
2.4不良反應對照組患者出現頭痛3例,手指震顫2例,藥物減量后不良反應消失;觀察組患者未出現嚴重不良反應。
3討論
CVA屬于一種特殊類型的哮喘,也是一種隱匿型哮喘,以慢性、持續性、頑固性咳嗽為主要臨床特征[7],其臨床癥狀常不典型,缺乏特異性,易誤診為支氣管炎或上呼吸道感染,而誤診、誤治會貽誤最佳治療時機,使患者病情反復發作,部分患者會發展成典型性哮喘[8-9]。CVA的病理基礎是氣道高反應性(airway hyper reactivity,AHR)、氣道炎癥、肺動靜脈擴張及氣道痙攣等,是由肥大細胞、嗜酸粒細胞(eosinophil,EOS)及T細胞等炎性細胞參與,白三烯(leukotrienes,LTs)介導的慢性非特異性變態反應性呼吸道炎癥[10]。LTs是體內非常重要的炎性遞質,可使支氣管平滑肌收縮、支氣管變窄、增強AHR、支氣管黏膜水腫、增加支氣管黏膜分泌黏液、降低纖毛清除能力,導致氣道重構[11]。白三烯受體拮抗劑(leukotrienes receptor antagonist,LTRAs)可通過與體內受體競爭結合,對半胱氨酰白三烯(cysteinyl leukotrienes,Cys-LTs)導致的炎癥發揮作用,LTRAs對于哮喘的治療具有重要作用,且耐受性較好[12]。
沙美特羅替卡松粉吸入劑是一種長效β2腎上腺素受體激動劑聯合糖皮質激素的吸入劑[13];沙美特羅可擴張支氣管平滑肌,解除支氣管痙攣,可長時間保持支氣管舒張,降低AHR,激活糖皮質激素受體,與氟替卡松聯合具有協同作用,可增加氟替卡松受體的敏感性,提高氟替卡松的抗炎活性[14];吸入激素主要作用于肺部,無口服激素的不良反應,已成為治療肺部疾病的主要給藥途徑[15]。
孟魯司特片是一種常用的新型高選擇性半胱氨酰白三烯受體拮抗劑(cysteinyl leukotrienes receptor antagonist,Cys-LTRAs),是目前最強效的LTRAs,已廣泛應用于CVA的治療中[16]。孟魯司特片可競爭性抑制氣道平滑肌中Cys-LTs多肽的活性,阻斷Cys-LTs與其受體特異性結合,減輕CVA速發和遲發相變態反應,降低毛細血管通透性,減少EOS在氣道聚集、浸潤及活化[4];抑制炎性細胞成熟、黏附、聚集,減輕氣道的局部炎性反應,減少呼吸道黏膜分泌量;抑制肥大細胞與LTs產生的致喘、致炎作用[17],減輕氣管炎癥,抑制支氣管痙攣及AHR,擴張支氣管[4],從而達到治療、控制病情及減少復發的目的[18-19]。孟魯司特片可控制大多數CVA患者的臨床癥狀,且臨床療效確切[20]。有研究表明,孟魯司特片聯合沙美特羅替卡松對CVA的臨床癥狀緩解效果優于單獨用藥,且治愈時間短[21]。
本研究結果顯示,觀察組患者臨床療效優于對照組,FEV1、FEV1/FVC、PEF高于對照組,CRT、CDT短于對照組,RR低于對照組。表明孟魯司特片聯合沙美特羅替卡松粉吸入劑治療CVA的臨床療效確切,可有效改善患者肺功能,縮短患者CRT及CDT,降低復發率,且安全性較高,值得臨床推廣應用。
參考文獻
[1]Niimi A.Cough and Asthma[J].Current Respiratory Medicine Reviews,2011,7(1):47-54.
[2]Tajiri T,Niimi A,Matsumoto H,et al.Prevalence and clinical relevance of allergic rhinitis in patients with classic asthma and cough variant asthma[J].Respiration,2014,87(3):211-218.
[3]Ohta K,Yamaguchi M,Akiyama K,et al.Japanese guideline for adult asthma[J].Allergol Int,2011,60(2):115-145.
[4]Takemura M,Niimi A,Matsumoto H,et al.Clinical,physiological and anti-inflammatory effect of montelukast in patients with cough variant asthma[J].Respiration,2012,83(4):308-315.
[5]Okunishi K,Peters-Golden M.Leukotrienes and airway inflammation[J].Biochimica Et Biophysica Acta,2011,1810(11):1096-1102.
[6]中華醫學會呼吸病學分會哮喘學組.咳嗽的診斷與治療指南(2009版)[J].中華結核和呼吸雜志,2009,7(5):407-413.
[7]Ohkura N,Fujimura M,Nakade Y,et al.Heightened cough response to bronchoconstriction in cough variant asthma[J].Respirology,2012,17(6):964-968.
[8]Krishnan JA,Bender BG,Wamboldt FS,et al.Adherence to inhaled corticosteroids:an ancillary study of the Childhood Asthma Management Program clinical trial[J].J Allergy Clin Immunol,2012,129(1):112-118.
[9]Lougheed MD,Turcotte SE,Fisher T.Cough variant asthma:lessons learned from deep inspirations[J].Lung,2012,190(1):17-22.
[10]Lipińska-Ojrzanowska A,Wiszniewska M,Walusiak-Skorupa J.Cough-variant asthma:a diagnostic dilemma in the occupational setting[J].Occup Med (Lond),2015,65(2):165-168.
[11]Rely K,McQuire SE,Alexandre PK,et al.Cost effectiveness of treatment with salmeterol/fluticasone compared to montelukast for the control of persistent asthma in children[J].Value Health,2011,14(5 Suppl 1):S43-S47.
[12]Pedersen SE,Hurd SS,Lemanske RF J,et al.Global strategy for the diagnosis and management of asthma in children 5 years and younger[J].Pediatr Pulmonol,2011,46(1):1-17.
[13]Paggiaro P,Patel S,Nicolini G,et al.Stepping down from high dose fluticasone/salmeterol to extrafine BDP/F in asthma is cost-effective[J].Respir Med,2013,107(10):1531-1537.
[14]Liu ZW,Yue F,Gao FY,et al.Research on the molecular mechanism of Seretide treatment to asthma disease[J].Eur Rev Med Pharmacol Sci,2012,16(12):1701-1706.
[15]Kagohashi K,Satoh H,Ohara G,et al.Long-term safety of budesonide/formoterol for the treatment of elderly patients with bronchial asthma[J].Exp Ther Med,2014,7(4):1005-1009.
[16]Ilarraza R,Wu Y,Adamko DJ.Montelukast inhibits leukotriene stimulation of human dendritic cells in vitro[J].Int Arch Allergy Immunol,2012,159(4):422-427.
[17]Fujimura M.Pathophysiology,diagnosis and treatment of cough variant asthma[J].Rinsho Byori,2014,62(5):464-470.
[18]Souza FC,Gobbato NB,Maciel RG,et al.Effects of corticosteroid,montelukast and iNOS inhibition on distal lung with chronic inflammation[J].Respir Physiol Neurobiol,2013,185(2):435-445.
[19]Tamaoki J,Yokohori N,Tagaya E,et al.Comparable effect of a leukotriene receptor antagonist and long-acting beta2-adrenergic agonist in cough variant asthma[J].Allergy Asthma Proc,2010,31(5):78-84.
[20]Keast SL,Thompson D,Farmer K,et al.Impact of a prior authorization policy for montelukast on clinical outcomes for asthma and allergic rhinitis among children and adolescents in a state Medicaid program[J].J Manag Care Spec Pharm,2014,20(6):612-621.
[21]Saito N,Itoga M,Tamaki M,et al.Cough variant asthma patients are more depressed and anxious than classic asthma patients[J].J Psychosom Res,2015,79(1):18-26.
(本文編輯:李潔晨)
Clinical Effect of Montelukast Combined With Salmeterol and Fluticas Inhalant on Cough Variant Asthma
WANGYong,YANGYa-chun,LIUYong.
DepartmentofRespiratoryMedicine,RailwayHospitalofXinyi,Xinyi221400,China
【Abstract】ObjectiveTo observe the clinical effect of montelukast combined with salmeterol and fluticas inhalant on cough variant asthma.MethodsA total of 112 patients with cough variant asthma were selected in the Railway Hospital of Xinyi from 2011 to 2014,and they were divided into control group and observation group according to visiting sequence,each of 56 cases.Patients of control group received salbutamol aerosol after admission,while patients of observation group received montelukast combined with salmeterol and fluticas inhalant;both groups continuously treated for 8 weeks.Clinical effect,index of lung function(including FEV1,FEV1/FVC and PEF),cough relief time(CRT),cough disappearance time(CDT),recurrence rate and incidence of adverse reactions were compared between the two groups.ResultsThe clinical effect of observation group was statistically significantly better than that of control group(P<0.05).No statistically significant differences of FEV1,FEV1/FVC or PEF was found between the two groups before treatment(P>0.05),while FEV1,FEV1/FVC and PEF of observation group were statistically significantly higher than those of control group(P<0.05).CRT and CDT of observation group were statistically significantly shorter than those of control group,and recurrence rate of observation group was statistically significantly lower than that of control group(P<0.05).Of control group,3 cases occurred headache,2 cases occurred finger fremitus;no one of observation group occurred any serious adverse reactions.ConclusionMontelukast combined with salmeterol and fluticas inhalant has certain clinical effect in treating cough variant asthma,can effectively improve the lung function,shorten the CRT and CDT,reduce the recurrence rate,and is safe.
【Key words】Asthma;Montelukast;Salmeterol and fluticas inhalant;Treatment outcome
【中圖分類號】R 562.25
【文獻標識碼】B
doi:10.3969/j.issn.1008-5971.2016.04.035
(收稿日期:2016-01-10;修回日期:2016-04-15)

