混合二甲苯系統熱力學平衡組成的計算
趙 巖,任冬梅,夏云生,李新華,包德才
(渤海大學化學化工學院,遼寧 錦州 121013)
?
有機化工與催化
混合二甲苯系統熱力學平衡組成的計算
趙巖*,任冬梅,夏云生,李新華,包德才
(渤海大學化學化工學院,遼寧 錦州 121013)
摘要:研究混合二甲苯模型中3種組分在不同溫度下的熱力學平衡組成,依據狀態函數特點,分別計算各平衡的標準摩爾反應熵變、標準摩爾反應焓變及標準摩爾吉布斯自由能變,進而計算平衡組成。結果表明,在該混合二甲苯理想氣體模型中,反應溫度由298.15 K升至1 023.15 K,混合二甲苯中3個平衡組成隨著反應溫度升高呈如下變化規律:間二甲苯含量y(MX)由0.599 0逐漸降至0.496 3,降幅達17.15%;鄰二甲苯含量y(OX)由0.162 6逐漸升至0.275 9,升幅達69.68%;對二甲苯含量y(PX)由0.238 4緩慢升至0.242 6,而后逐漸降至0.227 7,最大值出現在423.15 K,最大降幅僅為6.14%,表明升高溫度不利于提高對二甲苯的平衡組成。
關鍵詞:化學熱力學;混合二甲苯;平衡組成;對二甲苯;異構化
CLC number:TQ241.1+3;O643.12Document code: AArticle ID: 1008-1143(2016)05-0075-06
混合二甲苯是間二甲苯(MX)、鄰二甲苯(OX)和對二甲苯(PX)構成的混合體系,如指定了某一溫度和壓力,則該系統的狀態函數不再變化,即此時系統處于熱力學動態平衡,其組分含量是定值。當混合二甲苯看作理想氣體或理想液體混合物時,可以對系統熱力學性質進行演繹推理,為深入開發混合二甲苯轉化工藝提供重要的熱力學基礎數據。工業合成對二甲苯的工藝(如甲苯歧化、烷基轉移和汽油裂解等)主要產物為混合二甲苯[1-3],因此要設計高效分離設備,對生產操作(如冷凝、氣化、閃蒸、精餾、吸收、萃取、結晶和吸附)進行優化。本文對混合二甲苯系統熱力學平衡組成的計算進行研究。
1模型建立


圖1 理想二甲苯混合系統模型Figure 1 Ideal model of xylene mixed system
2計算過程
計算平衡組成熱力學方程式及計算路線:

3結果與討論


表1 二甲苯的標準摩爾恒壓熱容與溫度的關系[4-5]
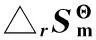
表2 不同反應溫度下二甲苯平衡體系的標準摩爾反應熵變(T)


表3 不同反應溫度下二甲苯平衡體系的標準摩爾反應焓變(T)


表4 不同反應溫度下二甲苯平衡體系的標準摩爾反應吉布斯自由能變(T)
從表4可以看出,反應溫度低于673.15 K,僅有鄰二甲苯向對二甲苯的異構化反應可以自發進行,且隨著反應溫度升高,自發進行的趨勢越來越弱;反應溫度高于673.15 K,上述3個異構化反應均不能自發進行,且隨著反應溫度升高,自發程度越來越難。
3.5平衡常數及平衡組成

幅為17.15%;鄰二甲苯含量隨著反應溫度升高逐漸提高,增幅為69.68%;對二甲苯含量隨著反應溫度升高先增后降,降幅為6.14%。從熱力學角度看,反應溫度變化對二甲苯含量影響較小,而升高溫度更有利于間二甲苯向鄰二甲苯異構。因此,若要提高對二甲苯選擇性,需要從反應動力學著手[9-11]。選擇合適的擇形催化劑并適當改性[12-23]以及設計新的分離工藝[24]或合成路線[25]均至關重要。

表5 不同反應溫度下二甲苯平衡體系的氣相組成
4結論
(1) 通過對混合二甲苯熱力學平衡組成的計算可知,間二甲苯含量隨著反應溫度升高逐漸降低,鄰二甲苯含量隨著反應溫度升高逐漸提高,對二甲苯含量隨著反應溫度升高先增加后降低,但降幅較小。升高溫度有利于促進間二甲苯向鄰二甲苯異構,而對二甲苯含量變化不大。若要提高對二甲苯選擇性,需從選擇催化劑、分離工藝和新合成路線入手。
(2) 混合二甲苯系統熱力學平衡組成的計算結果將為產品質量控制提供至關重要的熱力學參數和多元相平衡數據,經過對某些參數的適當修正,該方法適用于其他理想混合體系的熱力學平衡計算。
參考文獻:
[1]Chen N Y,Kaeding W W,Dwyer F G.Para-directed aromatic reactions over shape-selective molecular sieve zeolite catalysts[J].Journal of the American Chemical Society,1979,101(22):6783-6784.
[2]Paul B Weisz.Molecular shape selective catalysis[J].Pure and Applied Chemistry,1980,52:2091-2103.
[3]Kaeding W W,Chu C,Young B,et al.Shape-selective reactions with zeolite catalysts Ⅱ.Selective disproportionation of toluene to produce benzene and p-xylene[J].Journal of Catalysis,1981,69:392-398.
[4]John A Dean.Lange’s Handbook of Chemistry[M].United States of America:McGraw-Hill Book Company,1967.
[5]Robert C Weast.Handbook of Chemistry and Physics[M].70th ed.London:Wolfe Medical Publications Ltd.,1989.
[6]Rozanska X,van Santen R A,Hutschka F,et al.A periodic DFT study of intramolecular isomerization reactions of toluene and xylenes catalyzed by acidic mordenite[J].Journal of the American Chemical Society,2001,123(31):7655-7667.
[7]康承琳,龍軍,周震寰,等.二甲苯異構化的反應化學[J].石油學報(石油加工),2012,28(4):533-537.
Kang Chenglin,Long Jun,Zhou Zhenhuan,et al.Reaction chemistry of xylene isomerization[J].Acta Petrolei Sinica(Petroleum Processing Section),2012,28(4):533-537.
[8]李玲玲,聶小娃,宋春山,等.H-ZSM-5分子篩催化二甲苯異構化的反應機理[J].物理化學學報,2013,29(4):754-762.
Li Lingling,Nie Xiaowa,Song Chunshan,et al.Isomerization mechanism of xylene catalyzed by H-ZSM-5 molecular sieve[J].Acta Physico-Chimica Sinica,2013,29(4):754-762.
[9]李玉光,胡軍.二甲苯在改性ZSM-5沸石上異構化動力學[J].化學物理學報,1996,9(4):339-344.
Li Yuguang,Hu Jun.Kinetics of xylene isomerization on modified ZSM-5 zeolites[J].Chinese Journal of Chemical Physics,1996,9(4):339-344.
[10]李增和,劉秀英,馬麗景,等.HZSM-5催化劑上間二甲苯異構化反應動力學研究[J].北京化工大學學報,1997,24(4):67-70.
Li Zenghe,Liu Xiuying,Ma Lijing,et al.Reaction kinetics of m-xylene isomerization over zeolite ZSM-5[J].Journal of Beijing University of Chemical Technology,1997,24(4):67-70.
[11]陳金仙,張春,婁玥蕓,等.HZSM-5分子篩上間二甲苯異構化反應動力學研究[J].南京工業大學學報(自然科學版),2012,34(3):66-69.
Chen Jinxian,Zhang Chun,Lou Yueyun,et al.Reaction kinetics simulation of m-xylene isomerization over HZSM-5 zeolite catalyst[J].Journal of Nanjing Universsity of Technology(Natural Science Edition),2012,34(3):66-69.
[12]Zhao Y,Wu H Y,Tan W,et al.Effect of metal modification of HZSM-5 on catalyst stability in the shape-selective methylation of toluene[J].Catalysis Today,2010,156(1/2):69-73.
[13]Zhao Y,Tan W,Wu H Y,et al.Effect of Pt on stability of nano-scale ZSM-5 catalyst for toluene alkylation with methanol into p-xylene[J].Catalysis Today,2011,160(1):179-183.
[14]Ahn J H,Kolvenbach R,Al-Khattaf S S,et al.Methanol usage in toluene methylation with medium and large pore zeolites[J].ACS Catalysis,2013,3:817-825.
[15]Lu P,Fei Z Y,Li L,et al.Effects of controlled SiO2deposition and phosphorus and nickel doping on surface acidity and diffusivity of medium and small sized HZSM-5 for para-selective alkylation of toluene by methanol[J].Applied Catalysis A:General,2013,453:302-309.
[16]Tan W,Liu M,Zhao Y,et al.Para-selective Methylation of toluene with methanol over nano-sized ZSM-5 catalysts:synergistic effects of surface modifications with SiO2,P2O5and MgO[J].Microporous and Mesoporous Materials,2014,196:18-30.
[17]Magdolna R M,Márton K,Szilvia K,et al.Transformation of ethylbenzene-m-xylene feed over MCM-22 zeoliteswith different acidities[J].Applied Catalysis A:General,2014,476:19-25.
[18]Zepeda T A,Pawelec B,Infantes-Molina A,et al.Ortho-xylene hydroisomerization under pressure on HMS-Ti mesoporous silica decorated with Ga2O3nanoparticles[J].Fuel,2015,158:405-415.
[19]Zepeda T A,Infantes-Molina A,Díaz de Leon J N,et al.Synthesis and characterization of Ga-modified Ti-HMS oxidematerials with varying Ga content[J].Journal of Molecular Catalysis A:Chemical,2015,397:26-35.
[20]Wu Y L,Li J C,Chai Y M,et al.Synergetic effect of H-ZSM-5/silicalite-1@Pt/Al2O3core-shell catalyst to enhance the selective hydrogenation of p-xylene[J].Journal of Membrane Science,2015,496:70-77.
[21]Ding W J,Li Hui,Pfeifer P,et al.Crystallite-pore network model of transport and reaction of multicomponent gas mixtures in polycrystalline microporous media[J].Chemical Engineering Journal,2014,254:545-558.
[22]Wu Q M,Wang X,Meng X J,et al.Organotemplate-free,seed-directed,and rapid synthesis of Al-rich zeolite MTT with improved catalytic performance in isomerization of m-xylene[J].Microporous and Mesoporous Materials,2014,186:106-112.
[23]Ahn J H,Kolvenbach R,Gutiérrez O Y,et al.Tailoring p-xylene selectivity in toluene methylation on medium pore-size zeolites[J].Microporous and Mesoporous Materials,2015,210:52-59.
[24]Gonalves J C,Rodrigues A E.Simulated moving bed reactor for p-xylene production:adsorbent and catalyst homogeneous mixture[J].Chemical Engineering Journal,2014,258:194-202.
[25]Zhang Y Y,Li Y F,Chen L,et al.A newcatalytic process for the synthesis of para-xylene through benzene methylation with CH3Br[J].Catalysis Communications,2014,54:6-10.
Calculation of thermodynamic equilibrium compositions of mixed xylenes
Zhao Yan*, Ren Dongmei, Xia Yunsheng, Li Xinhua, Bao Decai
(College of Chemistry and Chemical Engineering, Bohai University, Jinzhou 121013, Liaoning, China)
Abstract:The thermodynamic equilibriums of three components of mixed xylenes model under different temperatures were researched.Based on the characteristics of the state function,three thermodynamic functions (T) and (T) of three equilibriums components under different temperatures were calculated,respectively.And then the equilibrium compositions were calculated.The results showed that in the ideal gas model of mixed xylene,the change rules of the three equilibrium compositions with the increase of reaction temperatures from 298.15 K to 1 023.15 K were as follows:the y(MX)of m-xylene contents reduced gradually from 0.599 0 to 0.496 3 with the drop of 17.15%;the y(OX)of o-xylene contents enhanced gradually from 0.162 6 to 0.275 9 with the increase of 69.68%;the y(PX)of p-xylene contents increased slowly from 0.238 4 to 0.242 6,and then decreased gradually to 0.227 7.Its maximum of 0.242 6 at 423.15 K with the biggest drop of 6.14% was obtained,which indicated that increasing temperature was unfavorable to improving balance composition of p-xylene.
Key words:chemical thermodynamics; mixed xylenes; equilibrium composition; p-xylene; isomerization
收稿日期:2016-03-16
基金項目:渤海大學博士啟動基金(BSQD201416,BSQD201417)資助項目;國家自然科學基金項目(21076026);遼寧省教育廳項目(L2013430)
作者簡介:趙巖,1976年生,男,遼寧省錦州市人,博士,講師,研究方向為多相催化。
doi:10.3969/j.issn.1008-1143.2016.05.015 10.3969/j.issn.1008-1143.2016.05.015
中圖分類號:TQ241.1+3;O643.12
文獻標識碼:A
文章編號:1008-1143(2016)05-0075-06
通訊聯系人:趙巖。

