單囊胚冷凍復(fù)蘇周期中囊胚時(shí)期及質(zhì)量與臨床結(jié)局相關(guān)分析
倪曉蓓,王珊珊,張寧媛,孫海翔
(南京大學(xué)醫(yī)學(xué)院附屬鼓樓醫(yī)院生殖醫(yī)學(xué)中心,江蘇南京 210008)
?
·臨床研究·
單囊胚冷凍復(fù)蘇周期中囊胚時(shí)期及質(zhì)量與臨床結(jié)局相關(guān)分析
倪曉蓓,王珊珊,張寧媛,孫海翔*
(南京大學(xué)醫(yī)學(xué)院附屬鼓樓醫(yī)院生殖醫(yī)學(xué)中心,江蘇南京210008)
目的比較囊胚時(shí)期及囊胚質(zhì)量在單囊胚冷凍復(fù)蘇周期中與臨床結(jié)局的關(guān)系。方法回顧性分析2014年1 月至2015 年12月在本中心進(jìn)行單囊胚冷凍復(fù)蘇周期的患者,總例數(shù)為243例;分析研究囊胚時(shí)期及囊胚質(zhì)量對(duì)臨床結(jié)局的影響。結(jié)果囊胚時(shí)期及囊胚質(zhì)量與臨床結(jié)局有相關(guān)性:囊胚移植D5與D6植入率(61.14%vs.44.00%)和臨床妊娠率(60.62%vs.44.00%)有顯著性差異(P<0.05);優(yōu)質(zhì)囊胚與非優(yōu)質(zhì)囊胚植入率(66.94%vs.47.90%)和臨床妊娠率(66.94%vs.47.06%)亦有顯著性差異(P<0.01)。患者的年齡、體重指數(shù)、基礎(chǔ)激素水平、內(nèi)膜厚度、流產(chǎn)率、異位妊娠率等均無統(tǒng)計(jì)學(xué)意義(P>0.05)。結(jié)論單囊胚冷凍復(fù)蘇時(shí)可以優(yōu)先考慮D5優(yōu)質(zhì)囊胚移植。
單囊胚冷凍復(fù)蘇;囊胚時(shí)期;囊胚質(zhì)量;臨床結(jié)局
Methods:The data of 243 cryopreserved single blastocyst transfer cycles at the Reproductive Center from January 2014 to December 2015 were retrospectively analyzes. The clinical outcomes were compared among the blastocysts with different development periods and scoring parameters.
Results:Totally,the development periods & quality of blastocysts significantly impacted clinical pregnancy (P<0.05). The implantation rate(61.14% vs. 44.00%)and pregnancy rate (60.62% vs. 44.00%)of Day 5 and Day 6 blastocysts were significantly different (P<0.05). The implantation rate(66.94% vs. 47.90%)and pregnancy rate(66.94% vs. 47.06%)of high quality and non-quality blastocysts were also significantly different (P<0.01). There was no significance in age,body mass index,baseline hormone levels,endometrial thickness of the patients among the groups (P>0.05).
Conclusions:The transfer with high quality blastocysts on Day5 should be prioritized in cryopreserved single blastocyst transfer cycles.
(JReprodMed2016,25(10):926-931)
隨著胚胎培養(yǎng)技術(shù)的發(fā)展,囊胚培養(yǎng)的成功率得到改善,現(xiàn)已證實(shí)囊胚移植較卵裂期胚胎移植的臨床妊娠率高[1-3],Papanikolaou等[4]研究表明高評(píng)分的卵裂期胚胎培養(yǎng)至囊胚后移植相比高評(píng)分卵裂期胚胎移植組具有更好的妊娠率和活產(chǎn)率,近年來越來越多的輔助生殖中心開展單囊胚移植以期降低多胎率的同時(shí)維持可觀的妊娠率。囊胚冷凍前的人工皺縮技術(shù)的發(fā)展使得冷凍復(fù)蘇囊胚移植獲得了與新鮮囊胚移植相似的活產(chǎn)率[5-6],大大推進(jìn)了囊胚冷凍復(fù)蘇技術(shù)在輔助生殖技術(shù)中的應(yīng)用,但是如何在冷凍復(fù)蘇周期中挑選最佳發(fā)育潛能的囊胚還有待進(jìn)一步研究。目前沿用的囊胚質(zhì)量評(píng)分系統(tǒng)由Gardner等[7]提出,基于內(nèi)細(xì)胞團(tuán)、滋養(yǎng)外胚層形態(tài)和擴(kuò)張等級(jí)三個(gè)參數(shù)。關(guān)于囊胚質(zhì)量和囊胚時(shí)期對(duì)囊胚移植臨床結(jié)局的影響目前還存在爭(zhēng)議[8-10],而如何選擇最佳的囊胚進(jìn)行移植關(guān)乎每對(duì)不孕夫婦的妊娠結(jié)局,這在單囊胚移植技術(shù)的推廣中尤為重要,因此,本研究旨在評(píng)估單囊胚冷凍復(fù)蘇周期中囊胚時(shí)期、囊胚質(zhì)量與臨床結(jié)局的相關(guān)性,以期為臨床應(yīng)用提供有效的參考。
資料和方法
一、研究對(duì)象
2014年1月至2015年12月在本院生殖醫(yī)學(xué)中心行輔助生殖治療的單囊胚冷凍復(fù)蘇周期患者。為減小其他因素的影響,患者納入研究標(biāo)準(zhǔn):(1)在本中心行 IVF 助孕的患者,且為單囊胚冷凍復(fù)蘇周期;(2)年齡≤40歲;(3)夫妻雙方染色體核型分析正常;(4)內(nèi)膜準(zhǔn)備方案為HRT方案,排除中重度子宮內(nèi)膜異位癥、子宮畸形、子宮多發(fā)性息肉患者。其中D5囊胚移植組193例;D6囊胚移植組50例。所有患者夫妻雙方均理解并簽署知情同意書。
二、研究方法
1. 治療方案:用常規(guī)促排卵方案進(jìn)行卵巢刺激,B超監(jiān)測(cè)卵泡生長(zhǎng)發(fā)育,待主導(dǎo)卵泡直徑達(dá)18~20 mm當(dāng)晚注射HCG 250 μg(默克雪蘭諾,德國(guó))或HCG10 000 IU(麗珠醫(yī)藥),間隔 36 h左右在B超引導(dǎo)下嚴(yán)格按照無菌要求穿刺取卵。
2. IVF:均采用短時(shí)受精,將卵丘復(fù)合物采集后置 IVF (Vitrolife,Sweden)微滴中培養(yǎng)3 h,加入精子(1×104/卵),通過觀察第二極體的形成判斷受精。
3.受精、卵裂的觀察和冷凍胚胎的篩選:受精后置于G1(Vitrolife,Sweden)序貫培養(yǎng)液中培養(yǎng),16~18 h觀察原核形成。取卵后48 h觀察卵裂情況,于72 h選擇優(yōu)質(zhì)胚胎移植、冷凍或?qū)ay3的分裂期胚胎轉(zhuǎn)入囊胚培養(yǎng)液G2 (Vitrolife,Sweden)微滴中,繼續(xù)培養(yǎng)至Day5,觀察囊胚發(fā)育情況。根據(jù)Garnder囊胚分級(jí)法對(duì)囊胚進(jìn)行分級(jí)[6],本中心將評(píng)分≥3BC或≥3CB的囊胚定為可冷凍囊胚。
4.囊胚冷凍與復(fù)蘇冷凍基礎(chǔ)液:本中心配置方法:羥乙基哌嗪乙磺酸(HEPES)緩沖的合成人輸卵管液(mHTF)中添加20%人血清蛋白(SSS,SAGE);冷凍液Ⅰ:mHTF中添加10%乙二醇(EG,Sigma,Germany)+10%二甲基亞砜(DMSO,Sigma,Germany);冷凍液Ⅱ:mHTF中添加20%EG+20%DMSO+0.3 mol/L蔗糖(Sigma,Germany)。解凍液Ⅰ:mHTF中添加20%SSS+1 mol/L蔗糖;解凍液Ⅱ:mHTF中添加20%SSS+0.5 mol/L蔗糖;解凍液Ⅲ:mHTF中添加20% SSS+0.25 mol/L蔗糖;解凍液Ⅳ:mHTF中添加20%SSS。囊胚冷凍:將囊胚置于 G2 培養(yǎng)液(Vitrolife,Sweden) 30 μl 培養(yǎng)液滴內(nèi),利用激光破膜儀系統(tǒng)進(jìn)行人工皺縮,待囊腔近乎完全皺縮后移入基礎(chǔ)冷凍平衡液,2 min后,先移入冷凍液Ⅰ中10 min,再移入冷凍液Ⅱ,60 s內(nèi)裝載至冷凍載桿的塑料薄膜片上并直接投入液氮中保存。胚胎復(fù)蘇:37℃下,直接將載桿的塑料薄膜片迅速浸入解凍液Ⅰ,使胚胎滑落入培養(yǎng)液中。1 min后,按照濃度梯度遞減的順序,將胚胎在室溫下依次移入解凍液Ⅱ、解凍液Ⅲ、解凍液Ⅳ,并分別停留3、5、5 min,最后在37℃下將解凍液Ⅳ中的胚胎移入胚胎培養(yǎng)液(G2),于37℃、5%O2、6%CO2培養(yǎng)箱中培養(yǎng)2 h并觀察囊胚恢復(fù)情況后移植。
5. 子宮內(nèi)膜的準(zhǔn)備:患者從月經(jīng)周期第 1天開始持續(xù)用補(bǔ)佳樂6 mg/d,月經(jīng)周期第14天日開始監(jiān)測(cè)子宮內(nèi)膜厚度,當(dāng)子宮內(nèi)膜厚度超過8 mm,肌肉注射黃體酮60 mg,每日一次,囊胚于黃體酮注射后6 d移植。
6. 妊娠結(jié)局的判定、黃體支持及妊娠判定:胚胎移植后常規(guī)黃體支持,移植后14 d檢測(cè)血HCG陽性,4~6周B超檢查見孕囊及原始心管搏動(dòng)視為臨床妊娠。
三、統(tǒng)計(jì)學(xué)方法
結(jié) 果
一、患者基本資料及囊胚時(shí)期、囊胚質(zhì)量與臨床結(jié)局的關(guān)系
兩組患者一般情況比較無統(tǒng)計(jì)學(xué)差異(P>0.05)(表1)。
二、不同囊胚時(shí)期移植組患者臨床妊娠結(jié)局比較
囊胚時(shí)期(按D5囊胚移植、D6囊胚移植分組)、囊胚質(zhì)量(按優(yōu)質(zhì)囊胚移植、非優(yōu)質(zhì)囊胚移植分組)與臨床妊娠率的關(guān)系有統(tǒng)計(jì)學(xué)意義(P<0.05)D5囊胚移植者的種植率和臨床妊娠率均高于D6囊胚移植者(P<0.05),而兩組患者雙胎率、畸形率、流產(chǎn)率、異位妊娠率等情況均無統(tǒng)計(jì)學(xué)差異(P>0.05)(表2)。
三、不同質(zhì)量囊胚移植組患者臨床妊娠結(jié)局比較
優(yōu)質(zhì)的囊胚移植組植入率、臨床妊娠率均高于非優(yōu)質(zhì)囊胚組(P<0.05),而兩組的雙胎率、畸形率、流產(chǎn)率、異位妊娠率無統(tǒng)計(jì)學(xué)差異(P>0.05)(表3)。
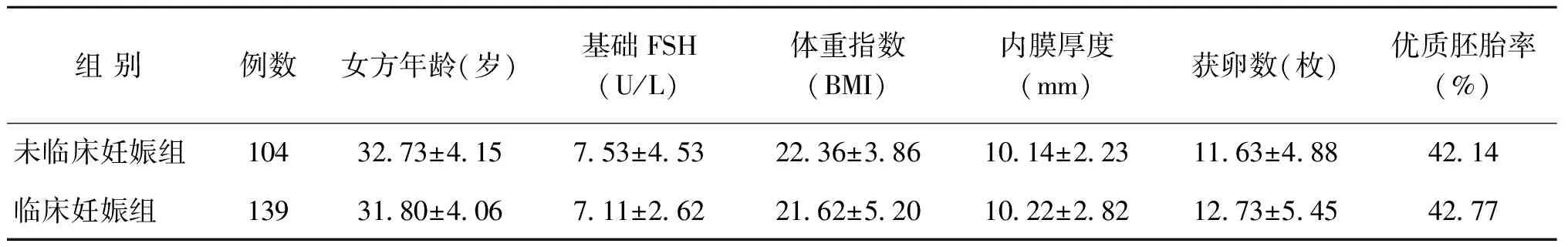
表1 患者一般情況比較(x-±s)
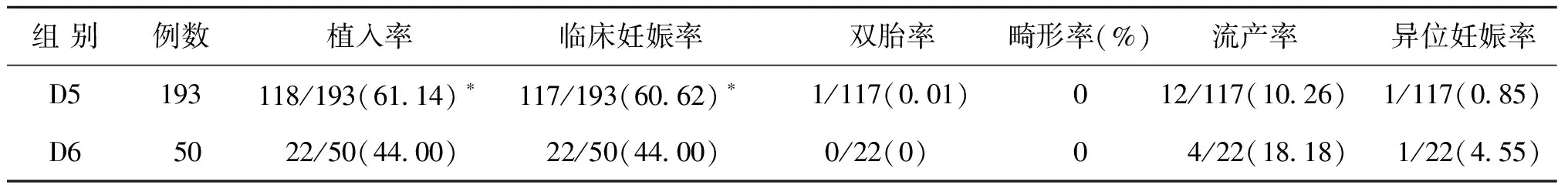
表2 D5囊胚移植組與D6囊胚移植組患者臨床妊娠結(jié)局比較[n,(%)]
注:與D6囊胚移植組比較,*P<0.05
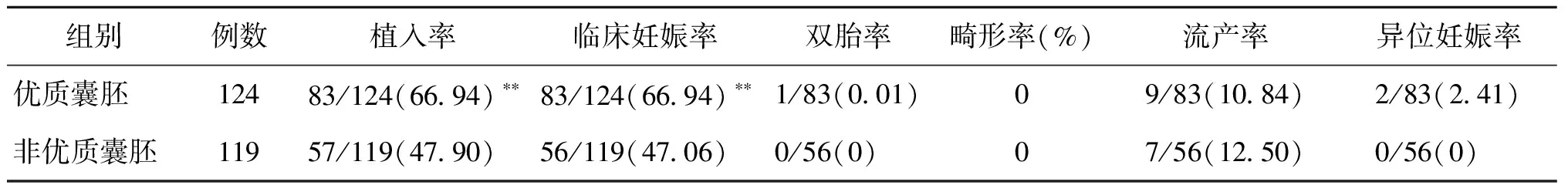
表3 優(yōu)質(zhì)囊胚移植組與非優(yōu)質(zhì)囊胚移植組患者臨床妊娠結(jié)局比較[n,(%)]
注:與非優(yōu)質(zhì)囊胚移植組比較,**P<0.01
討 論
隨著人類輔助生殖技術(shù)的發(fā)展,多胎已成為其重要的并發(fā)癥之一,多胎會(huì)增加孕婦妊娠及新生兒出生的風(fēng)險(xiǎn),文獻(xiàn)報(bào)道在輔助生殖治療中多胎率的發(fā)生與女方年齡、治療過程等密切相關(guān),其中一個(gè)關(guān)鍵的因素就是目前治療中用多胚胎移植的方法來提高妊娠率的臨床策略,因此在臨床實(shí)踐中推廣單胚胎移植是解決這一問題的有效方法,而囊胚期的單胚胎移植將是較好的選擇[11-12]。有文獻(xiàn)報(bào)道囊胚移植與子宮內(nèi)膜的同步性更好,從而有利于提高妊娠率[13],此外,囊胚的形成需要延長(zhǎng)體外培養(yǎng)時(shí)間,從而可以篩選出發(fā)育潛能更好的胚胎用于移植或冷凍,更有利于妊娠,囊胚培養(yǎng)挑選出了最佳發(fā)育潛能的胚胎。有報(bào)道顯示達(dá)到冷凍標(biāo)準(zhǔn)的頂級(jí)第三天胚胎有59%具有基因?qū)W的異常,而達(dá)到冷凍標(biāo)準(zhǔn)的頂級(jí)囊胚35%有基因?qū)W的異常[14],開展囊胚移植淘汰了部分具有遺傳缺陷的胚胎,這將有助于提高妊娠率,Papanikolaou等[2,15]報(bào)道在鮮胚移植中單囊胚移植較單分裂胚移植有更高的活產(chǎn)率。但是這也不能完全避免染色體異常而形態(tài)學(xué)正常的囊胚被移植[16],結(jié)合種植前基因?qū)W診斷可以有效的篩選染色體正常的囊胚進(jìn)行移植,提高植入率和妊娠率[17-18]。同時(shí)囊胚培養(yǎng)也可能會(huì)增加某些患者的風(fēng)險(xiǎn),導(dǎo)致無可移植胚胎的情況[13]。也有學(xué)者對(duì)兩種時(shí)期移植的出生兒結(jié)局做了調(diào)查,單因素回歸分析的結(jié)果發(fā)現(xiàn)囊胚移植、促排卵方案、供卵都可能增加同卵雙生的比率,而在多因素回歸分析中發(fā)現(xiàn)囊胚移植與同卵雙生的發(fā)生率更相關(guān),但是具體機(jī)制還有待研究[19]。在輔助生殖中同卵雙生的比率在1.2%~8.9%[19],本結(jié)果由于受樣本量的局限,還未有相關(guān)的發(fā)現(xiàn),后期會(huì)繼續(xù)觀察研究。因此,關(guān)于胚胎移植時(shí)期的選擇還是要根據(jù)患者情況進(jìn)行,目前在輔助生殖臨床工作中,由于一些特殊情況的需要如預(yù)防嚴(yán)重OHSS的發(fā)生、患者鮮胚移植日陰道感染等,能否開展單囊胚冷凍復(fù)蘇周期移植成為廣大患者和工作人員關(guān)注的焦點(diǎn),本研究旨在評(píng)估單囊胚冷凍復(fù)蘇周期中囊胚時(shí)期、囊胚質(zhì)量與臨床結(jié)局的相關(guān)性,以期為臨床單囊胚冷凍復(fù)蘇周期的推廣提供有效的參考。
關(guān)于囊胚質(zhì)量與妊娠結(jié)局的關(guān)系的研究還存在爭(zhēng)議[20-23]。目前沿用的囊胚質(zhì)量評(píng)分系統(tǒng)由Gardner等[7]提出,基于內(nèi)細(xì)胞團(tuán)、滋養(yǎng)外胚層形態(tài)和擴(kuò)張等級(jí)三個(gè)參數(shù)。一些研究者認(rèn)為內(nèi)細(xì)胞團(tuán)等級(jí)與臨床妊娠率呈正相關(guān),內(nèi)細(xì)胞團(tuán)質(zhì)量越好妊娠結(jié)局越好[24],然而,Hill等[25]和Thompson等[26]通過研究表明,在新鮮移植周期和冷凍復(fù)蘇周期中滋養(yǎng)外胚層質(zhì)量越好則臨床妊娠率和活產(chǎn)率越高。此外,Ahlstrom等[27]和Abbeel等[28]研究表明依據(jù)囊胚腔擴(kuò)張的等級(jí)可以預(yù)測(cè)單囊胚移植后的臨床結(jié)局。在本研究納入的243例單囊胚冷凍復(fù)蘇移植患者中,均是用常規(guī)促排卵方案的IVF患者,依據(jù)患者的知情同意開展單囊胚冷凍復(fù)蘇移植。本研究表明在單囊胚冷凍復(fù)蘇周期中囊胚質(zhì)量與臨床結(jié)局的關(guān)系有統(tǒng)計(jì)學(xué)差異,內(nèi)細(xì)胞團(tuán)和滋養(yǎng)層質(zhì)量?jī)?yōu)質(zhì)的囊胚無論是植入率還是臨床妊娠率均高于非優(yōu)質(zhì)囊胚組,而兩組的多胎率、畸形率、流產(chǎn)率無統(tǒng)計(jì)學(xué)差異,提示在臨床實(shí)踐中應(yīng)優(yōu)先挑選優(yōu)質(zhì)囊胚進(jìn)行移植。至于為什么優(yōu)質(zhì)囊胚具有更好的臨床結(jié)局相關(guān)機(jī)制還有待于進(jìn)一步研究。有報(bào)道提示優(yōu)質(zhì)囊胚的非整倍體率要低于非優(yōu)質(zhì)囊胚[29-30],而非整倍體的比率與流產(chǎn)的發(fā)生相關(guān)[31-32],值得注意的是在本結(jié)果中優(yōu)質(zhì)囊胚移植組的流產(chǎn)率與非優(yōu)質(zhì)囊胚移植組的差異有趨勢(shì)但無統(tǒng)計(jì)學(xué)意義(10.84% vs. 12.50%),可能與本研究樣本量偏小有關(guān),預(yù)計(jì)將進(jìn)行后續(xù)的追蹤。
關(guān)于究竟是移植D5囊胚還是D6囊胚更具優(yōu)勢(shì)目前還存在爭(zhēng)議[8-10],Dessolle等[33]認(rèn)為D5囊胚移植的健康嬰兒出生率顯著高于 D6囊胚移植,建議避免 D6 移植,認(rèn)為囊胚時(shí)期是關(guān)乎妊娠結(jié)局的重要因素。Muthukumar等[34]研究發(fā)現(xiàn)D5囊胚移植的臨床妊娠率和植入率均高于 D6 囊胚移植;王雪等[35]發(fā)現(xiàn)D5囊胚的種植率、活產(chǎn)率高于D6囊胚,但無統(tǒng)計(jì)學(xué)差異。本研究結(jié)果表明在冷凍復(fù)蘇周期中囊胚時(shí)期影響妊娠結(jié)局,D5囊胚移植者的種植率和臨床妊娠率均高于D6囊胚移植者,而兩組患者年齡、BMI值、基礎(chǔ)內(nèi)分泌、流產(chǎn)率、異位妊娠率等情況均無統(tǒng)計(jì)學(xué)差異,與已報(bào)道的研究結(jié)果[34]是一致的,因此針對(duì)這部分患者在選擇囊胚移植時(shí)可以優(yōu)選D5囊胚從而改善臨床妊娠結(jié)局。有研究認(rèn)為D6囊胚的發(fā)育潛能要低于D5囊胚,這可能是D5囊胚移植結(jié)局好于D6囊胚的原因之一[36]。也有文獻(xiàn)提示D5囊胚的非整倍體率要低于D6囊胚(53.3% vs. 60.2%),但是這種差異無統(tǒng)計(jì)學(xué)意義[29]。因此,相關(guān)機(jī)制還有待進(jìn)一步研究。
綜上所述,對(duì)于單囊胚冷凍復(fù)蘇周期的患者,在選擇囊胚移植時(shí),為了使患者利益最大化,在臨床實(shí)踐中應(yīng)該診視每一枚形成的囊胚,同時(shí)根據(jù)實(shí)際情況可以首先優(yōu)選D5優(yōu)質(zhì)囊胚進(jìn)行移植,本研究的結(jié)果將為推動(dòng)冷凍復(fù)蘇周期單囊胚移植提供有效的臨床依據(jù)。但是由于本研究是小樣本的回顧性研究,囊胚選擇的數(shù)據(jù)主要基于本中心現(xiàn)有的標(biāo)準(zhǔn),結(jié)果還存在局限性,待后續(xù)研究。
[1]Aziminekoo E,Mohseni Salehi MS,Kalantari V,et al. Pregnancy outcome after blastocyst stage transfer comparing to early cleavage stage embryo transfer[J]. Gynecological Endocrinology,2015,31:880-884.
[2]Papanikolaou EG,Camus M,Kolibianakis EM,et al. In vitro fertilization with single blastocyst-stage versus single cleavage-stage embryos[J]. N Engl J Med,2006,354:1139-1146.
[3]Santos MJ,Mercader A,Galan A,et al. Implantation rates after two,three,or five days of embryo culture[J]. Placenta,2003,24(Suppl B):S13-19.
[4]Papanikolaou EG,Elke DH,Greta V,et al. Live birth rate is significantly higher after blastocyst transfer than after cleavage-stage embryo transfer when at least four embryos are available on day 3 of embryo culture. A randomized prospective study[J]. Hum Reprod,2005,20:3198-3203.
[5]Evans J,Hannan NJ,Edgell TA,et al. Fresh versus frozen embryo transfer:backing clinical decisions with scientific and clinical evidence[J]. Hum Reprod Update,2014,20:808-821.
[6]Roy TK,Bradley CK,Bowman MC,et al. Single-embryo transfer of vitrified-warmed blastocysts yields equivalent live-birth rates and improved neonatal outcomes compared with fresh transfers[J]. Fertil Steril,2014,101:1294-1301.
[7]Gardner DK,Lane M,Stevens J,et al. Blastocyst score affects implantation and pregnancy outcome:towards a single blastocyst transfer[J]. Fertil Steril,2000,73:1155-1158.
[8]Liebermann J,Tucker MJ. Comparison of vitrification and conventional cryopreservation of day 5 and day 6 blastocysts during clinical application[J]. Fertil Steril,2006,86:20-26.
[9]Levens ED,Gustofson RL,Hennessy S,et al.Implantation rate and pregnancy outcomes are significantly improved in cryopreserved blastocyst transfers when frozen at day 5 compared to day 6[J]. Fertil Steril,2006,86:S256-S257.
[10]Richter KS,Shipley SK,Ingrid MV,et al. Cryopreserved embryo transfers suggest that endometrial receptivity may contribute to reduced success rates of later developing embryos[J]. Fertil Steril,2006,86:862-866.
[11]Bhattacharya S,Kamath MS. Reducing multiple births in assisted reproduction technology[J]. Best Pract Res Clin Obstet Gynaecol,2014,28:191-199.
[12]Messinis IE,Ekaterini D. What is the most relevant standard of success in assisted reproduction? Should BESST really be the primary endpoint for assisted reproduction?[J]. Hum Reprod,2004,19:1933-1935.
[13]Maheshwari A,Hamilton M,Bhattacharya S. Should we be promoting embryo transfer at blastocyst stage?[J/OL]. Reprod Biomedi Online,2015,32:142-146.
[14]Magli MC,Jones GM,Gras L,et al. Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro[J]. Hum Reprod,2000,15:1781-1786.
[15]Papanikolaou EG,Kolibianakis EM,Tournaye H. Live birth rates after transfer of equal number of blastocysts or cleavage-stage embryos in IVF. A systematic review and meta-analysis[J]. Hum Reprod,2008,23:91-99.
[16]Fragouli E,Alfarawati S,Spath K,et al. Morphological and cytogenetic assessment of cleavage and blastocyst stage embryos[J]. Mol Hum Reprod,2014,20:117-126.
[17]Forman EJ,Upham KM,Cheng M,et al.Comprehensive chromosome screening alters traditional morphology-based embryo selection:a prospective study of 100 consecutive cycles of planned fresh euploid blastocyst transfer[J]. Fertil Steril,2013,100:718-724.
[18]Scott RT,Upham KM,F(xiàn)orman EJ,et al. Blastocyst biopsy with comprehensive chromosome screening and fresh embryo transfer significantly increases in vitro fertilization implantation and delivery rates:a randomized controlled trial[J]. Fertil Steril,2013,100:697-703.
[19]Luke B,Brown MB,Wantman E,et al.. Factors associated with monozygosity in assisted reproductive technology pregnancies and the risk of recurrence using linked cycles[J]. Fertil Steril,2014,101:683-689.
[20]Racowsky C,Combelles CM,Nureddin A,et al. Day 3 and day 5 morphological predictors of embryo viability[J/OL]. Reprod Biomed Online,2003,6:323-331.
[21]Kresowik JD,Sparks AE,Van Voorhis BJ. Clinical factors associated with live birth after single embryo transfer[J]. Fertil Steril,2012,98:1152-1156.
[22]甄璟然,王雪,孫正怡,等. 復(fù)蘇單囊胚移植周期中囊胚形態(tài)學(xué)評(píng)分對(duì)臨床妊娠的影響[J]. 生殖醫(yī)學(xué)雜志,2014,23:7-10.
[23]王雅琴,楊菁,徐望明,等. 低等級(jí)胚胎囊胚培養(yǎng)及凍融周期移植后臨床結(jié)局分析[J]. 生殖醫(yī)學(xué)雜志,2015,24:220-224.
[24]Goto S,Kadowaki T,Tanaka S,et al. Prediction of pregnancy rate by blastocyst morphological score and age,based on 1,488 single frozen-thawed blastocyst transfer cycles[J]. Fertil Steril,2011,95:948-952.
[25]Hill MJ,Richter KS,Heitmann RJ,et al. Trophectoderm grade predicts outcomes of single-blastocyst transfers[J]. Fertil Steril,2013,99:1283-1289.e1.
[26]Thompson SM,Onwubalili N,Brown K,et al. Blastocyst expansion score and trophectoderm morphology strongly predict successful clinical pregnancy and live birth following elective single embryo blastocyst transfer (eSET):a national study[J]. J Assist Reprod Genet,2013,30:1577-1581.
[27]Ahlstrom A,Westin C,Wikland M,et al.Prediction of live birth in frozen-thawed single blastocyst transfer cycles by pre-freeze and post-thaw morphology[J]. Hum Reprod,2013,28:1199-1209.
[28]Abbeel EVD,Balaban B,Ziebe S,et al. Association between blastocyst morphology and outcome of single-blastocyst transfer[J/OL]. Reprod Biomed Online,2013,27:353-361.
[29]Antonio C,Laura R,Danilo C,et al. Correlation between standard blastocyst morphology,euploidy and implantation:an observational study in two centers involving 956 screened blastocysts[J]. Hum Reprod,2014,29:1173-1181.
[30]Samer A,Elpida F,Pere C,et al. The relationship between blastocyst morphology,chromosomal abnormality,and embryo gender[J]. Fertil Steril,2011,95:520-524.
[31]Stephenson MD,Awartani KA,Robinson WP. Cytogenetic analysis of miscarriages from couples with recurrent miscarriage:a case-control study[J]. Hum Reprod,2002,17:446-451.
[32]Fritz B,Hallermann C,Olert J,et al. Cytogenetic analyses of culture failures by comparative genomic hybridisation (CGH)-Re-evaluation of chromosome aberration rates in early spontaneous abortions[J]. Eur J Hum Gene,2001,9:539-547.
[33]Dessolle L,F(xiàn)reour T,Ravel C,et al. Predictive factors of healthy term birth after single blastocyst transfer[J]. Hum Reprod,2011,26:1220-1226.
[34]Muthukumar K,Kamath MS,Mangalaraj AM,et al. Comparison of clinical outcomes following vitrified warmed day 5/6 blastocyst transfers using solid surface methodology with fresh blastocyst transfers[J]. J Hum Reprod Sci,2013,6:59-64.
[35]王雪,甄璟然,孫正怡,等. 囊胚發(fā)育速度對(duì)凍融移植結(jié)局的影響[J]. 生殖醫(yī)學(xué)雜志,2014,23:356-360.
[36]Voorhis BJ,Dokras A. Delayed blastocyst transfer:is the window shutting?[J]. Fertil Steril,2008,89:31-32.
[編輯:谷炤]
Development periods and quality of blastocysts impact clinical outcomes in cryopreserved single blastocyst transfer cycles
NI Xiao-bei,WANG Shan-shan,ZHANG Ning-yuan,SUN Hai-xiang*
CenterofReproductiveMedicine,DrumTowerHospitalAffiliatedtoNanjingUniversitySchoolofMedicine,Nanjing210008
Objective:To assess cycle outcome among the blastocysts with different development periods and scoring parameters in cryopreserved single blastocyst transfer cycles.
Cryopreserved single blastocyst transfer cycle;Development periods of blastocyst;Blastocysts quality;Clinical outcomes
10.3969/j.issn.1004-3845.2016.10.014
2016-03-24;
2016-04-24
倪曉蓓,女,江蘇南通人,碩士,研究實(shí)習(xí)員,主要從事生殖醫(yī)學(xué)研究.(*

