基于CT圖像紋理分析評價結直腸癌肝轉移新輔助治療后療效的價值
胡飛翔,胡婷丹,童 彤,彭衛軍
復旦大學附屬腫瘤醫院放射診斷科,復旦大學上海醫學院腫瘤學系,上海 200032
基于CT圖像紋理分析評價結直腸癌肝轉移新輔助治療后療效的價值
胡飛翔,胡婷丹,童 彤,彭衛軍
復旦大學附屬腫瘤醫院放射診斷科,復旦大學上海醫學院腫瘤學系,上海 200032

彭衛軍,教授,復旦大學博士研究生導師,現任復旦大學附屬腫瘤醫院放射診斷科主任,中國抗癌協會腫瘤影像專業委員會候任主任委員,上海醫學會放射診斷專科委員會副主任委員,上海市抗癌協會腫瘤影像專業委員會主任委員,中華醫學會放射學分會乳腺學組副組長。
目的:探討治療前基線CT門靜脈期圖像的直方圖分析預測結直腸癌肝轉移(colorectal liver metastasis,CRLM) 新輔助治療后療效的價值。方法:選取34例CRLM患者,共計132枚病灶,經FOLFOX(氟尿嘧啶+亞葉酸鈣+奧沙利鉑)、FOLFIRI (氟尿嘧啶+亞葉酸鈣+伊立替康)或CapeOX(奧沙利鉑+卡培他濱或卡培他濱單用)方案化療。所有患者均接受至少兩次常規腹部平掃加增強三期CT掃描,于化療前4周內行CT基線掃描,化療開始后2~3個月內行第2次掃描以評估療效。對患者門靜脈期CT圖像進行直方圖分析,依據實體腫瘤療效評價標準(Response Evaluation Criteria in Solid Tumors,RECIST)(Version 1.1)進行療效評估,獲得相應轉移瘤的紋理參數,比較緩解與非緩解組患者治療前基線CT直方圖參數的差異。采用受試者工作特征(receiver operating characteristic,ROC)曲線分析法計算各參數預測緩解的曲線下面積(area under curve,AUC)、靈敏度、特異度、陽性預計值、陰性預計值、準確率及截斷值。由兩名放射科醫師達成一致意見后勾畫感興趣區。結果:34例患者中,緩解組21例,非緩解組13例。緩解組的均值、方差、偏度和百分位數(10%、50%、90%、99%)低于非緩解組,差異有統計學意義(P<0.05);但峰度值和1%百分位數無顯著差異(P=0.769、0.06)。90th百分位數在截斷值為167時具有較高的準確率(81.82%),此時靈敏度、特異度、陽性預計值、陰性預計值及AUC分別為74.42%、95.65%、96.65%、66.97%和0.854。結論:CT門靜脈期直方圖分析對預測CRLM患者新輔助療效具有潛在價值。
紋理分析;直方圖;結直腸癌肝轉移
目前,結直腸癌(colorectal cancer,CRC)是男性第三高發、女性第二高發的腫瘤[1],且15%~25%的患者在診斷為CRC時已發生肝轉移。然而,CRC原發病灶根治性切除手術后仍有15%~25%的患者發生肝轉移,其中約85%無法做到根治性切除[2-6],因此CRC肝轉移(colorectal liver metastasis,CRLM)也成為CRC患者的最主要死亡原因(60%~71%)[7]。對于無法進行根治性手術切除的CRC患者,往往采用姑息性化療。對于CRC確診后合并同期肝轉移患者,若原發病灶無出血、無梗阻癥狀或無穿孔,除外在技術上容易切除的肝轉移灶且不存在不良預后的患者,均建議采用新輔助治療[8-11]。全身化療方案包括:FOLFOX (氟尿嘧啶+亞葉酸鈣+奧沙利鉑)、FOLFIRI (氟尿嘧啶+亞葉酸鈣+伊立替康)、CapeOX (奧沙利鉑+卡培他濱或卡培他濱單用)或FOLFOXIRI (氟尿嘧啶+亞葉酸鈣+奧沙利鉑+伊立替康)[12-15]。但一線化療后,仍有約50%的CRC患者表現為無緩解甚至疾病進展[16]。對于這部分患者,臨床醫師可嘗試使用貝伐單抗或西妥昔單抗等靶向治療藥物。為減少化療對肝臟手術的不利影響,新輔助化療原則上不超過6個周期[17],一般建議2~3個月內完成并進行手術[18]。
CRLM新輔助化療后的療效預測對指導臨床制訂后續治療方案至關重要。CT可在形態學上對CRLM進行療效評價[19]。圖像紋理分析是近年來新出現的圖像后處理技術,通過量化分析影像圖像像素灰度值的局部特征、變化規律及分布模式等紋理特征來反映感興趣區(region of interest, ROI)內生物組織結構的不均質性[20-21]。在過去有關肝臟輔助診斷的紋理分析研究中,多采用平掃圖像[22]。近期CT增強圖像紋理分析的應用逐漸廣泛,增強掃描可提供病灶的血供情況及病灶內部血流變化特征[23]。影像圖像紋理分析可提供更多肉眼無法觀察到的圖像信息[24]。灰度直方圖分析是紋理分析中的一部分,可幫助量化腫瘤內部的異質性,在腫瘤診斷及療效評價中較常規形態學評估具有更多優勢[25-27]。
目前,關于CT增強掃描圖像的灰度直方圖分析預測CRLM新輔助化療后療效的研究鮮有報道。本研究采用門靜脈期CT增強圖像的灰度直方圖分析來判斷CRLM患者的近期療效,以期為臨床評估新輔助治療后療效并制訂后續治療方案提供有效輔助手段。
1 資料和方法
1.1 患者資料
收集復旦大學附屬腫瘤醫院2011年6月—2016年8月期間初診為CRLM的患者共34例,其中>1 cm的可測量病灶132枚。所有患者均接受CT平掃加三期增強檢查,并于基線檢查結束后進行全身化療。其中男性20例、女性14例;平均年齡(58.85±9.96)歲。患者的入選標準為:① 基線CT檢查時間在新輔助治療開始前4周內完成;② 患者在基線檢查前未接受任何CRC及肝臟病灶相關治療,包括手術、化療及放療等;③無結直腸外其他腫瘤相關病史;④ 第2次評估在新輔助治療后2~3個月內完成。
1.2 檢查方法
CT檢查采用SIEMENS公司Somatom Sensation型螺旋CT掃描儀,層厚8 mm,層距8 mm。患者檢查前常規禁食8~12 h,取平臥位,平掃后行增強掃描。使用碘海醇(300 gI/L)作為造影劑,按1.5 mL/kg體重計算給藥量。用高壓注射器經肘靜脈注射給藥,注射速率為2.5 mL/s。于造影劑注射后30 s、60 s及2~15 min分別行屏氣動脈期、門靜脈期及延遲掃描,掃描范圍包括所有病變區域。
1.3 圖像分析與評價標準
采用MaZda軟件(Version 4.6,Instytut Elektroniki)進行紋理分析[28]。圖像通過影像歸檔和通信系統(Picture Archiving and Communication Systems,PACS)下載并導入軟件中進行分析。對所有患者門靜脈期CT圖像進行ROI勾畫,由兩名放射科醫師達成一致意見后選取(兩名醫師分別具有3年和13年以上工作經驗),均不知道患者預后情況。ROI選擇于門靜脈期肝轉移瘤的最大層面進行,以>1 cm病灶為目標,沿病灶邊緣輪廓勾畫ROI,勾畫過程中盡可能避開大血管(包括門靜脈及肝靜脈等),生成ROI內的各個灰度直方圖參數值:均值(mean),方差(variance),偏度(skewness),峰度(kurtosis)及第1、20、50、90、99百分位數(1st、10th,50th,90th,99th percentile)。采用實體腫瘤療效評價標準(Response Evaluation Criteria in Solid Tumors,RECIST)(Version 1.1)進行療效評估[29],將完全緩解(complete response,CR)與部分緩解(partial response,PR)患者劃歸于緩解組,將疾病發展(progressive disease,PD)與疾病穩定(stable disease,SD)患者劃歸于非緩解組。然后對各紋理參數與療效情況進行統計學分析。
1.4 統計學處理
使用SPSS 21.0和MedCalc 12.7.2進行統計學分析。患者一般特征連續性變量符合正態分布者采用t檢驗,用表示;不符合正態分布者采用Mann-Whitney U檢驗,用中位值表示。對具有統計學意義的直方圖參數,進行受試者工作特征(receiver operating characteristic,ROC)曲線分析并計算相應的曲線下面積(area under curve, AUC)。截斷值采用最大約登指數計算:約登指數=靈敏度-(1-特異度)。
2 結 果
2.1 患者一般特征
根據RECIST標準,經新輔助治療后2~3個月,將患者分為治療后緩解組(A組,21例)和非緩解組(B組,13例)。患者接受全身化療方案包括:FOLFOX(10例)、FOLFIRI(14例)、CapeOX (10例)。詳見表1。
2.2 灰度直方圖各參數的診斷效能
結果顯示,CRLM患者經新輔助治療后2~3個月的療效評估可通過基線CT門靜脈期圖像灰度直方圖分析中的均值、方差、偏度及百分位數(10%、50%、90%、99%)進行有效預測。緩解組(圖1)中上述紋理參數顯著低于非緩解組(圖2) (P<0.05),但峰度值和1%百分位數在兩組之間無顯著差異(P=0.769、0.06)(表2)。采用ROC曲線分析CT門靜脈期直方圖各參數評價緩解組的診斷效能(圖3)。當90th百分位數≤167時,可獲得較高的準確率(81.82%),此時靈敏度、特異度、陽性預計值(positive predictive value,PPV)、陰性預計值(negative predictive value,NPV)及AUC分別為74.42%、95.65%、96.97%、66.67%和0.854 (表3)。
3 討 論
目前,肝轉移患者主要采用一線化療方案,但隨訪中發現約85%患者出現疾病進展[30],且只有不到50%的患者對一線化療敏感[31]。對于一線化療方案療效不理想者,可使用其他治療方案,包括加用分子靶向治療或聯用肝動脈灌注化療[32]。能否盡早預測新輔助化療療效對臨床制訂后續方案至關重要,已有研究評估了紋理分析評價療效的潛力。Ganeshan等[33]和Miles等[34]在研究CRC患者肝臟紋理情況時發現,肝臟的粗糙紋理與隱匿性惡性腫瘤和不良預后有關。本研究發現,低的均值、方差、偏度和百分位數(10%、50%、90%、99%)鑒別緩解與非緩解組具有統計學意義(P<0.05),而峰度值和1%百分位數鑒別緩解與非緩解組無顯著差異(P=0.769、0.06)。

圖1 新輔助化療后緩解組灰度直方圖及各參數
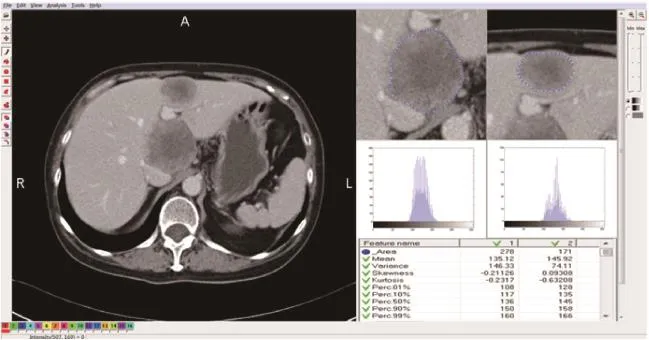
圖2 新輔助化療后非緩解組灰度直方圖及各參數
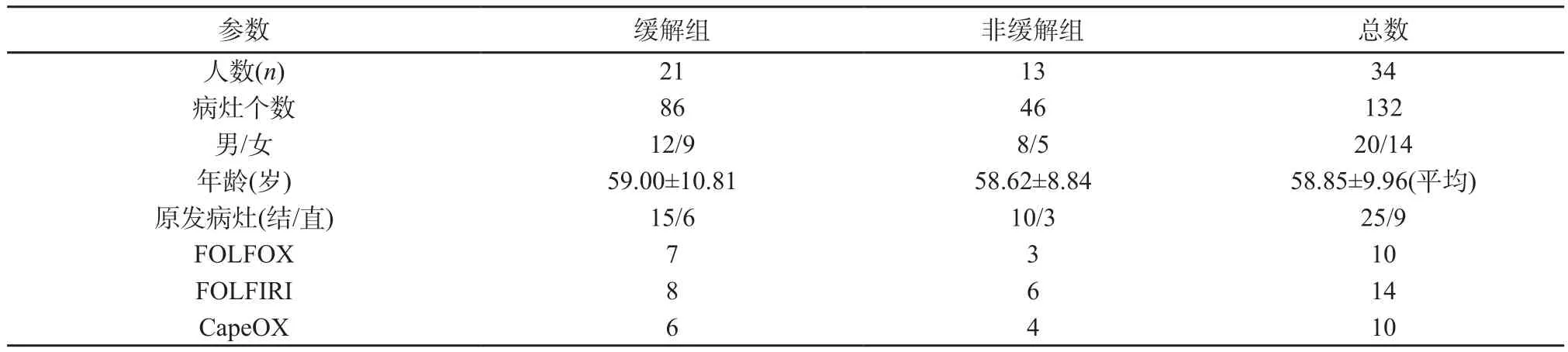
表1 患者一般特征

表2 緩解組(A組)與非緩解組(B組)之間門靜脈期CT圖像直方圖分析的比較

圖3 門靜脈期基線CT圖像直方圖各參數的ROC曲線
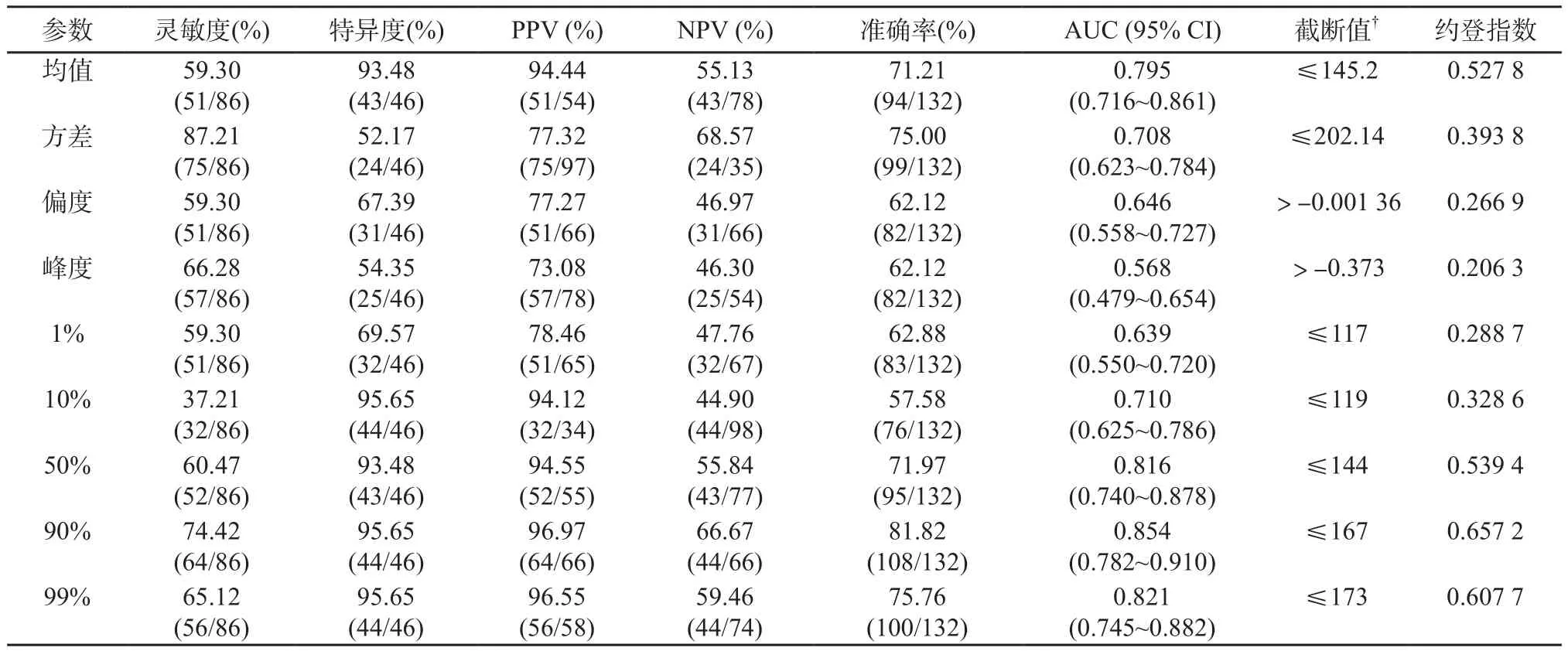
表3 紋理參數判斷緩解組與非緩解組的診斷效能
偏度的絕對值越大表示其分布形態的偏斜程度越大,方差表示平均值的變化情況,偏度和方差值的大小與同質均勻性成反比[35]。因此,高的偏度值和方差表示腫瘤具有較高的異質性。腫瘤的異質性是惡性腫瘤的公認特征,反映區域內高細胞密度、壞死、出血及黏液樣變[36]。腫瘤異質性是預后的重要因素,腫瘤異質性越高可能與腫瘤級別越高相關[37]。本研究結果與某些研究[34,38-39]相似,他們發現高偏度值和標準偏差代表腫瘤的異質性明顯,預示患者預后較差,與本研究中治療后非緩解組的偏度值和方差高相符。本研究顯示,90th百分位數具有較高的診斷準確率(81.82%),AUC值為0.854。90th百分位數是一個量度,表示包含了90% CT值的觀測數據,并剔除了直方圖中10%的最大值,這些最大值可能代表ROI勾畫過程中無法去除的腫瘤部分供血分支血管。因此,去除這部分10%的最大值,能更準確地反映腫瘤本身的真實強化特征。
目前,對于CT圖像紋理分析掃描期相的選擇并無固定標準。Goh等[40]采用動脈期圖像評估腎細胞癌患者酪氨酸激酶治療后腫瘤紋理的變化。另一些研究使用平掃來評估肝臟腫瘤[41]、肺癌[38]或食管癌[42]。然而,大多數研究[20]采用門靜脈期圖像的紋理來分析CRLM患者。本研究對門靜脈期圖像進行紋理分析,這是因為CRC患者監視隨訪期間,CT掃描常規需行門靜脈期檢查;且以往研究指出,CT門靜脈期觀察到的肝臟紋理與生物學性質相關,如總肝血流量和葡萄糖代謝,可作為CRC患者有價值的生物標記[33]。
本研究存在以下不足之處:首先,是一項回顧性研究,存在選擇偏倚。其次,樣本量較小且為單中心研究,需大樣本、多中心及前瞻性研究來驗證。第三,剔除了其他病理類型的CRC(如印戒細胞癌、黏液腺癌),最終選擇的患者均為結直腸腺癌,缺少其他病理類型的轉移。但其他病理類型例數非常少,因此對研究結果的影響甚微。第四,采用最大層面的方法勾畫ROI,沒有對整個病灶進行分析,可能會對結果造成一定的影響。但閱讀相關文獻,發現2D與3D紋理分析在CRLM患者治療前預測療效結果相似[43]。最后,由于統計數據量大及圖像后處理的限制,沒有進行其他期圖像的研究,沒有對紋理特征的其他參數(如灰度共生矩陣、游程矩陣、梯度模型、自回歸模型和基于傅里葉變換的紋理特征)進行分析,希望以后能對此進行深入研究。
4 結 語
CRLM患者基線CT門靜脈期灰度直方圖分析可幫助預測新輔助治療后是否緩解,具有一定的臨床應用價值。
[1] JEMAL A, BRAY F, CENTER M M, et al. Global cancer statistics [J]. CA Cancer J Clin, 2011, 61(2): 69-90.
[2] VIBERT E, CANEDO L, ADAM R. Strategies to treat primary unresectable colorectal liver metastases [J]. Semin Oncol, 2005, 32(6 Suppl 8): S33-S39.
[3] KEMENY N. Management of liver metastases from coloreetal cancer [J]. Oneology (Williston Park), 2006, 20(10): 1161-1176, 1179-1180, 1166-1185.
[4] LAU W, LAI E. Hepatic resection for colorectal liver metastases [J]. Singapore Med J, 2007, 48(7): 635-639.
[5] TANIAI N, AKIMARU K, YOSHIDA H, et al. Surgical treatment for better prognosis of patients with liver metastases from colorectal cancer [J]. Hepatogastroenterology, 2007, 54(78): 1805-1809.
[6] ARRU M, ALDRIGHETTI L, CASTOLDI R, et al. Analysis of prognostic factors influencing long-term survival after hepatic resection for metastatic colorectal cancer [J]. World J Surg, 2008, 32(1): 93-103.
[7] FOSTER J. Treatment of metastatic disease of the liver: a skeptic’s view [J]. Semin Liver Dis, 1984, 4(2): 170-179.
[8] TIMMERMAN R D, BIZEKIS C S, PASS H I, et al. Local surgical, ablative, and radiation treatment of metastases [J]. CA Cancer J Clin, 2009, 59(3): 145-170.
[9] BENOIST S, NORDLINGER B. Neoadjuvant treatment before resection of liver metastases [J]. Eur J Surg Oncol, 2007, 33(Suppl 2): S35-S41.
[10] REDDY S K, BARBAS A S, CLARY B M. Synchronous colorectal liver metastases: is it time to reconsider traditional paradigms of management? [J]. Ann Surg Oncol, 2009, 16(9): 2395-2410.
[11] POULTSIDES G A, SERVAIS E L, SALTZ L B, et al. Outcome of primary tumor in patients with synchronous stage Ⅳ colorectal cancer receiving combination chemotherapy without surgery as initial treatment [J]. J Clin Oncol, 2009, 27(20): 3379-3384.
[12] MEHTA N N, RAVIKUMAR R, COLDHAM C A, et al. Effect of preoperative chemotherapy on liver resection for colorectal liver metastases [J]. Eur J Surg Oncol, 2008, 34(7): 782-786.
[13] GRUENBERGER B, TAMANDL D, SCHUELLER J, et al. Bevacizumab, capecitabine, and oxaliplatin as neoadjuvant therapy for patients with potentially curable metastatic colorectal cancer [J]. J Clin Oncol, 2008, 26(11): 1830-1835.
[14] COSKUN U, BUYUKBERBER S, YAMAN E, et al. Xelox (capecitabine plus oxaliplatin) as neoadjuvantchemotherapy of unresectable liver metastases in colorectal cancer patients [J]. Neoplasma, 2008, 55(1): 65-70.
[15] CASSIDY J, CLARKE S, DIAZ-RUBIO E, et al. XELOX vs. FOLFOX-4 as first-line therapy for metastatic colorectal cancer: NO16966 updated results [J]. Br J Cancer, 2011, 105(1): 58-64.
[16] COLUCCI G, GEBBIA V, PAOLETTI G, et al. PhaseⅢ randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell’Italia Meridionale [J]. J Clin Oncol, 2005, 23(22): 4866-4875.
[17] NAKANO H, OUSSOULTZOGLOU E, ROSSO E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy [J]. Ann Surg, 2008, 247(1): 118-124.
[18] CHOTI M A. Chemotherapy-associated hepatotoxicity: do we need to be concerned? [J]. Ann Surg Oncol, 2009, 16(9): 2391-2394.
[19] MATSUHASHI N, TAKAHASHI T, KATO J, et al. Computed tomography evaluation of morphological changes, clinical response and survival in colorectal cancer liver metastasis treated by regorafenib: A case report [J]. Mol Clin Oncol, 2016, 5(6): 807-810.
[20] AHN S J, KIM J H, PARK S J, et al. Prediction of the therapeutic response after FOLFOX and FOLFIRI treatment for patients with liver metastasis from colorectal cancer using computerized CT texture analysis [J]. Eur J Radiol, 2016, 85(10): 1867-1874.
[21] RAJKUMAR V, GOH V, SIDDIQUE M, et al. Texture analysis of 125I-A5B7 anti-CEA antibody SPECT differentiates metastatic colorectal cancer model phenotypes and anti-vascular therapy response [J]. Br J Cancer, 2015, 112(12): 1882-1887.
[22] MOUGIAKAKOU S G, VALAVANIS I K, NIKITA A, et al. Differential diagnosis of CT focal liver lesions using texture features, feature selection and ensemble driven classifiers [J]. Artif Intell Med, 2007, 41(1): 25-37.
[23] HAFEEZ S, ALAM M S, SAJJAD Z, et al. Triphasic computed tomography (CT) scan in focal tumoral liver lesions [J]. J Pak Med Assoc, 2011, 61(6): 571-575.
[24] CASTELLANO G, BONILHA L, LI L M, et al. Texture analysis of medical images [J]. Clin Radiol, 2004, 59(12): 1061-1069.
[25] EMBLEM K E, NEDREGAARD B, NOME T, et al. Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps [J]. Radiology, 2008, 247(3): 808-817.
[26] CHOI Y S, KIM D W, LEE S K, et al. The added prognostic value of preoperative dynamic contrastenhanced MRI histogram analysis in patients with glioblastoma: Analysis of overall and progression-free survival [J]. AJNR Am J Neuroradiol, 2015, 36(12): 2235-2241.
[27] POPE W B, KIM H J, HUO J, et al. Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment [J]. Radiology, 2009, 252(1): 182-189.
[28] SZCZYPINSKI P M, STRZELECKI M, MATERKA A, et al. MaZda—a software package for image texture analysis [J]. Comput Methods Programs Biomed, 2009, 94(1): 66-76.
[29] EISENHAUER E A, THERASSE P, BOGAERTS J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) [J]. Eur J Cancer, 2009, 45(2): 228-247.
[30] GOLDBERG R M, SARGENT D J, MORTON R F, et al. A randomized controlled trial of fluorouracil plus leucovorin, irinotecan, and oxaliplatin combinations in patients with previously untreated metastatic colorectal cancer [J]. J Clin Oncol, 2004, 22(1): 23-30.
[31] TOURNIGAND C, ANDRE T, ACHILLE E, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: a randomized GERCOR study [J]. J Clin Oncol, 2004, 22(2): 229-237.
[32] KABBINAVAR F F, SCHULZ J, MCCLEOD M, et al. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase Ⅱ trial [J]. J Clin Oncol, 2005, 23(16): 3697-3705.
[33] GANESHAN B, MILES K A, YOUNG R C, et al. In search of biologic correlates for liver texture on portalphase CT [J]. Acad Radiol, 2007, 14(9): 1058-1068.
[34] MILES K A, GANESHAN B, GRIFFITHS M R, et al. Colorectal cancer: texture analysis of portal phase hepatic CT images as a potential marker of survival [J]. Radiology, 2009, 250(2): 444-452.
[35] MILES K A, GANESHAN B, HAYBALL M P. CT texture analysis using the filtration-histogram method: what do the measurements mean? [J]. Cancer Imaging, 2013, 13(3): 400-406.
[36] RAO R K. Prospective study of colorectal cancer in the West of Scotland: 10-year follow-up [J]. Br J Surg, 1990, 77(12): 1434.
[37] ECCLES S A, WELCH D R. Metastasis: recent discoveries and novel treatment strategies [J]. Lancet, 2007,369(9574): 1742-1757.
[38] GANESHAN B, ABALEKE S, YOUNG R C, et al. Texture analysis of non-small cell lung cancer on unenhanced computed tomography: initial evidence for a relationship with tumour glucose metabolism and stage [J]. Cancer Imaging, 2010, 10: 137-143.
[39] KATO H, KANEMATSU M, ZHANG X, et al. Computer-aided diagnosis of hepatic fibrosis: preliminary evaluation of MRI texture analysis using the finite difference method and an artificial neural network [J]. AJR Am J Roentgenol, 2007, 189(1): 117-122.
[40] GOH V, GANESHAN B, NATHAN P, et al. Assessment of response to tyrosine kinase inhibitors in metastatic renal cell cancer: CT texture as a predictive biomarker [J]. Radiology, 2011, 261(1): 165-171.
[41] HUANG Y L, CHEN J H, SHEN W C. Diagnosis of hepatic tumors with texture analysis in nonenhanced computed tomography images [J]. Acad Radiol, 2006, 13(6): 713-720.
[42] GANESHAN B, SKOGEN K, PRESSNEY I, et al. Tumour heterogeneity in oesophageal cancer assessed by CT texture analysis: preliminary evidence of an association with tumour metabolism, stage, and survival [J]. Clin Radiol, 2012,67(2): 157-164.
[43] LUBNER M G, STABO N, LUBNER S J, et al. CT textural analysis of hepatic metastatic colorectal cancer: pre-treatment tumor heterogeneity correlates with pathology and clinical outcomes [J]. Abdom Imaging, 2015, 40(7): 2331-2337.
Value of CT texture analysis in evaluating response to neoadjuvant chemotherapy for colorectal liver metastases
HU Feixiang, HU Tingdan, TONG Tong, PENG Weijun
(Department of Diagnostic Radiology, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China)
PENG Weijun E-mail: cjr.pengweijun@vip.163.com
Objective:To explore the value of histogram analysis of baseline CT portal images before treatment in predicting the response of patients with colorectal liver metastases (CRLMs) to neoadjuvant chemotherapy.Methods:Thirty-four patients (a total of 132 lesions) diagnosed with CRLM were retrospectively enrolled and underwent contrast-enhanced CT before and after neoadjuvant chemotherapy (FOLFOX, FOLFIRI or CapeOX ). All patients underwent pre-treatment CT baseline scan withinfour weeks for primary tumor assessment and a second CT scan in 2 to 3 months, for response evaluation. Histogram of CT portal images of patients with CRLM was analyzed and response was mainly assessed using Response Evaluation Criteria in Solid Tumors (RECIST) Version 1.1. The texture parameters of the metastatic tumor were analyzed statistically to fi nd the dif f erences in baseline CT histogram parameters between responding group and non-responding group before and after treatment. The receiver operating characteristic (ROC) curves were depicted to characterize each parameter value in evaluating the treatment outcomes. The optimal cutof f value (obtained according to the maximal Youden index = sensitivity + specif i city-1), the corresponding sensitivity, specif i city, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated. Regions of interests (ROIs) were manually drawn on the largest cross-sectional area of the primary lesions by two radiologists in consensus.Results:Twenty-one responding and 13 non-responding patients were evaluated. The values of mean, variance, skewness and percentile (10th, 50th, 90th, 99th) in responding group were much lower than those in non-responding group (P<0.05). The kurtosis and 1st percentile values between the two groups had no significant difference (P=0.769, P=0.06, respectively). The optimal cutoff value for the accurate identif i cation of responding patients was 167 for 90th percentile (74.42% sensitivity, 95.65% specif i city, 96.97% PPV, 66.67% NPV, 81.82% accuracy, and 0.854 area under curve, respectively).Conclusion:CT histogram analysis of baseline CT portal images before treatment can help to predict the response of patients with CRLM after neoadjuvant chemotherapy.
Texture analysis; Histogram; Colorectal liver metastasis
R445.3
A
1008-617X(2017)02-0106-08
2017-04-01)
國家自然科學基金項目(No:81501437)
彭衛軍 E-mail:cjr.pengweijun@vip.163.com

