miR-106b在鼻咽癌組織中表達及對鼻咽癌CNE-2細胞生物學特性的影響
周士霞,王海莉,王豪勛
(鄭州大學第二附屬醫院,鄭州 450014)
miR-106b在鼻咽癌組織中表達及對鼻咽癌CNE-2細胞生物學特性的影響
周士霞,王海莉,王豪勛
(鄭州大學第二附屬醫院,鄭州 450014)
目的 探討miR-106b在鼻咽癌組織中的表達及其對鼻咽癌CNE-2細胞生物學特性的影響。方法 選取68例份初治鼻咽癌患者的手術切除標本和45例份鼻咽炎患者的鼻咽部活檢組織標本,采用實時熒光定量PCR法檢測miR-106b表達,分析miR-106b表達與鼻咽癌患者臨床病理參數的關系。取體外培養的對數生長期人鼻咽癌細胞株CNE-2分為四組(1×106個/組)。miR-106b模擬物組、miR-106b抑制物組分別轉染miR-106b模擬物及miR-106b抑制物序列,陰性對照組轉染陰性對照序列,空白對照組不處理;采用實時熒光定量PCR技術檢測各組miR-106b表達,MTT法檢測各組細胞增殖情況(以吸光度值表示),流式細胞儀檢測各組細胞周期,Transwell試驗檢測各組細胞遷移和侵襲能力。結果 鼻咽癌及鼻咽炎組織miR-106b相對表達量分別為1.57±0.15、1.14±0.12,二者比較P<0.05; miR-106b相對表達量與鼻咽癌患者淋巴結轉移、侵犯頸動脈鞘和侵犯顱底有關(P均<0.05)。各組miR-106b相對表達量:miR-106b模擬物組高于miR-106b抑制物組、陰性對照組和空白對照組,miR-106b抑制物組低于陰性對照組和空白對照組,P均<0.05;各組細胞增殖情況:miR-106b模擬物組細胞接種后24、48、72和96 h時吸光度值均高于miR-106b抑制物組、陰性對照組和空白對照組,而miR-106b抑制物組低于陰性對照組和空白對照組,P均<0.05;各組細胞周期:miR-106b模擬物組S期細胞比例高于miR-106b抑制物組、陰性對照組和空白對照組,而miR-106b抑制物組細胞S期比例低于陰性對照組和空白對照組,P均<0.05;各組細胞遷移和侵襲情況:miR-106b模擬物組遷移和侵襲細胞數均高于miR-106b抑制物組、陰性對照組和空白對照組,且miR-106b抑制物組均低于陰性對照組和空白對照組,P均<0.05。結論 miR-106b在鼻咽癌組織中呈高表達; miR-106b過表達可促進鼻咽癌CNE-2細胞增殖、遷移和侵襲能力。
鼻咽癌;微小RNA-106b;CNE-2細胞;細胞增殖;細胞遷移
微小RNA(miRNA)是廣泛存在于生物體內的高度保守的短小RNA,在細胞增殖、分化、遷移等多種生物學過程中發展重要作用,且參與了多種惡性腫瘤的發生、侵襲、轉移過程[1~3]。miR-106b作為miRNA的重要成員,與原癌基因簇成員miR-17、miR-20有高度同源性,高表達于多種惡性腫瘤[4],且與腫瘤細胞增殖關系密切[5]。但有關miR-106b與鼻咽癌發病的相關性鮮有報道。本研究對鼻咽癌組織中miR-106b表達變化進行分析,并通過轉染miR-106b模擬物和抑制物,探討其對人鼻咽癌細胞株CNE-2增殖、遷移及侵襲能力的影響,以期為鼻咽癌發病機制的研究提供依據。
1 材料與方法
1.1 材料 選取68例份2014年2月有~2016年2月我院初治鼻咽癌患者[男43例、女25例,年齡27~81(47.6±11.5)歲;排除心肝腎等重要臟器嚴重功能障礙者以及糖尿病、惡性腫瘤患者]手術切除的癌組織標本,經病理學檢查確診。臨床分期:Ⅰ~Ⅱ期31例,Ⅲ~Ⅳ期37例;發生頸淋巴結轉移51例。選取同期行鼻咽部活檢的45例慢性鼻咽炎患者[男29例、女16例,年齡(48.0±12.1)歲]手術切除的正常胃咽部組織標本,經病理學檢查確診。鼻咽癌患者與鼻咽炎患者性別、年齡具有可比性(P均>0.05)。人鼻咽癌細胞株CNE-2購自中科院上海生科院細胞資源中心,FBS、DMEM培養基、Lipofectamine 2000轉染試劑盒均購自美國Invitrogen公司,MTT細胞增殖及細胞毒性檢測試劑盒購自上海威奧生物公司,Transwell小室購自美國Corning公司,實時熒光定量PCR儀購自美國Bio-rad公司,FACSCalibur流式細胞儀購自美國BD公司。TRIzol總RNA提取試劑盒購自加拿大BBI公司,逆轉錄試劑盒和PCR試劑盒均購自大連寶生物公司,miR-106b及內參U6引物、miR-106b模擬物、miR-抑制物和陰性對照均由上海生工公司設計合成。
1.2 鼻咽組織miR-106b表達檢測 采用實時熒光定量PCR法。取鼻咽癌及慢性鼻咽炎患者鼻咽部活檢組織,研磨后加入細胞裂解液進行裂解,用TRIzol總RNA提取試劑盒對總RNA進行提取,并檢測其純度,取A260/A280≥1.80的標本作為合格樣品。將總RNA逆轉錄為模板單鏈cDNA,以cDNA為模板,用PCR儀進行PCR擴增。PCR反應條件:94 ℃、60 s,92 ℃、30 s,56 ℃、30 s,74 ℃、30 s,連續進行38個循環。每個樣品均設置3個平行反應復孔。采用2-ΔΔCt法獲得不同組織中miR-106b相對表達量。分析miR-106b表達與鼻咽癌患者臨床病理參數的關系。
1.3 miR-106b對CNE-2細胞生物學特性影響的觀察
1.3.1 CNE-2細胞培養及分組處理 將CNE-2細胞培養于含100 μg/mL鏈霉素、100 U/mL青霉素和10% FBS中的DMEM完全培養基中,于5%CO2、37 ℃恒溫培養箱中培養,待細胞生長豐度達到80%以上時,胰酶消化后傳代培養。取對數生長期的CNE-2細胞,用無血清DMEM培養基重懸后,接種于6孔板,細胞密度5×104/mL,待細胞貼壁生長融合度達到50%以上后,用Lipofectamine 2000轉染試劑盒按照操作說明對細胞進行轉染。將細胞分成四組(1×106個/組):miR-106b模擬物組:轉染miR-106b模擬物序列:5′-TAAAGTGCTGACAGTGCAGAT-3′;miR-106b抑制物組:轉染miR-106b抑制物序列:5′-AUCUGCACUGUCAGCACUUUA-3′;陰性對照組:轉染陰性對照序列:5′-CAGUACUUUUGUGUAGUACAA-3′;空白對照組:不做任何處理。
1.3.2 miR-106b表達檢測 各轉染組細胞培養48 h后加入細胞裂解液進行裂解,采用實時熒光定量PCR法檢測miR-106b相對表達量。
1.3.3 細胞增殖率的測算 采用MTT法。取轉染24 h細胞,胰酶消化后,接種于96孔板內,細胞數2.0×103/孔,分別于接種后12、24、48、72和96 h時,將孔板中液體吸出,加入新鮮培養基和MTT,置于5% CO2、37 ℃恒溫箱中培養4 h,加入二甲基亞砜(DMSO),振蕩12 min后,用酶標儀進行檢測,取490 nm處吸光度(A)值。重復實驗3次,取平均值。
1.3.4 細胞周期檢測 采用流式細胞儀檢測。取各轉染組對數生長的細胞,接種于6孔板,細胞數1×105/孔,培養24 h后,胰酶消化后收集細胞,用預冷PBS洗滌3次,用乙醇固定,4 ℃下過夜孵育。加入碘化丙啶避光染色25 min,利用流式細胞儀檢測各轉染組細胞周期。重復實驗3次,取平均值。
1.3.5 細胞遷移能力及侵襲能力檢測 采用Transwell試驗。細胞遷移能力:取各組轉染后培養48 h細胞,胰酶消化后收集細胞,用無血清DMEM培養基重懸,調整細胞濃度為5×105個/mL,取200 μL細胞加入Transwell小室的上室,將含10%胎牛血清的DMEM培養基加入下室,于37 ℃培養箱中培養24 h,用甲醛進行固定,結晶紫染色后,用顯微鏡進行觀察,隨機選取5個高倍視野對穿膜細胞數進行計數,取平均數。重復實驗3次。細胞侵襲能力:將50 μg的Matrigel膠將Transwell小室底部膜包被,37 ℃成膠30 min,其余步驟同上。重復實驗3次,取平均值。
2 結果
2.1 鼻咽癌及鼻咽炎組織miR-106b表達比較 鼻咽癌組織、慢性鼻咽炎鼻咽組織中miR-106b相對表達量分別為1.57±0.15、1.14±0.12,二者比較差異有統計學意義(t=15.433,P<0.05)。miR-106b表達與鼻咽癌患者年齡、性別、臨床分期無關(P均>0.05),而與淋巴結轉移、侵犯頸動脈鞘和侵犯顱底有關(P均<0.05),詳見表1。
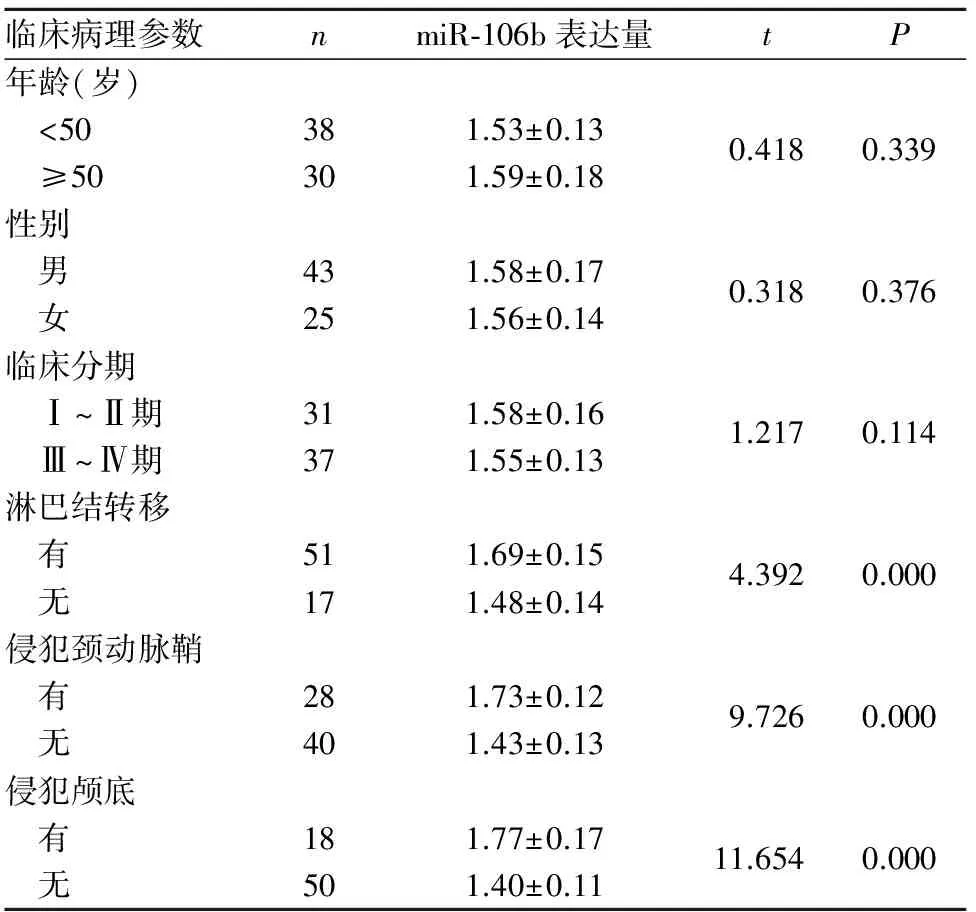
表1 miR-106b表達與鼻咽癌患者臨床病理參數的關系
2.2 miR-106b對鼻咽癌細胞生物學特性的影響
2.2.1 各組細胞中miR-106b表達比較 miR-106b模擬物組miR-106b相對表達量為1.82±0.16,顯著高于miR-106b抑制物組、陰性對照組和空白對照組,分別為1.07±0.09、1.31±0.13和1.33±0.14,且miR-106b抑制物組低于陰性對照組和空白對照組(P均<0.05)。
2.2.2 各組細胞增殖情況比較 見表2。
2.2.3 各組細胞周期比較 見表3。
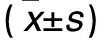
表2 各組細胞增殖情況比較
注:與空白對照組相比,*P<0.05;與陰性對照組相比,△P<0.05;與miR-106b抑制物組相比,#P<0.05。
2.2.4 各組細胞遷移和侵襲能力比較 見表4。
3 討論
miR-106b為miRNA的重要類型,參與了細胞增殖、分化、凋亡過程。研究發現, miR-106b過表達可促進肝細胞肝癌細胞增殖和轉移[7];miR-106b高表達于喉鱗狀細胞癌,通過負性調控APC基因而促進喉鱗狀細胞癌Hep-2細胞增殖[8]。本研究結果顯示,鼻咽癌組織中miR-106b相對表達量顯著高于慢性鼻咽炎組織,提示miR-106b可能參與了鼻咽癌的發生過程; miR-106b表達量與鼻咽癌患者年齡、 性別、原發灶大小和臨床分期無關,而與淋巴結轉移、侵犯頸動脈鞘和侵犯顱底有關,提示鼻咽癌患者發生頸淋巴結轉移、出現侵犯頸動脈鞘和侵犯顱底時,腫瘤組織中miR-106表達水平顯著升高,miR-106b可能與鼻咽癌局部侵襲和淋巴結及遠處轉移有關。
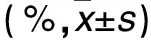
表3 各組細胞周期情況比較
注:與空白對照組相比,*P<0.05;與陰性對照組相比,△P<0.05;與miR-106b抑制物組相比,#P<0.05。
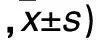
表4 各組細胞遷移和侵襲能力比較(個
注:與空白對照組相比,*P<0.05;與陰性對照組相比,△P<0.05;與miR-106b抑制物組相比,#P<0.05。
本研究為進一步研究miR-106b對鼻咽癌細胞株CNE-2增殖、遷移及侵襲能力的影響,利用細胞轉染技術,特異性上調或抑制細胞中miR-106b表達,結果顯示,miR-106b模擬物組細胞中miR-106b相對表達量顯著高于miR-106b抑制物組、陰性對照組和空白對照組,且miR-106b抑制物組明顯低于陰性對照組和空白對照組,說明轉染miR-106b模擬物或抑制物可特異性使細胞中miR-106b表達上調或抑制。miR-106b模擬物組細胞接種后24、48、72和96 h時A值均明顯高于miR-106b抑制物組、陰性對照組和空白對照組,而miR-106b抑制物組明顯低于陰性對照組和空白對照組,說明上調miR-106b可加速CNE-2細胞增殖,而抑制miR-106b表達則可抑制CNE-2細胞增殖,提示miR-106b參與了CNE-2細胞增殖過程。miR-106b模擬物組細胞S期比例明顯高于miR-106b抑制物組、陰性對照組和空白對照組,miR-106b抑制物組細胞S期比例明顯低于陰性對照組和空白對照組,說明miR-106b可能通過調控細胞周期而參與細胞增殖過程,與葉敏華等[9]研究結論相同。近年來文獻報道,miR-106b調控細胞周期的機制可能與調控了細胞周期中的一些相關因子有關[10]。有研究通過對調控細胞周期的相關基因進行篩選發現,p21/CDKN1A是miR-106b的直接作用靶位[11];miR-106b可通過激活轉錄因子E2F1而促使G1/S期的轉化[12]。本研究結果顯示,miR-106b模擬物組遷移細胞數和侵襲細胞數均明顯高于miR-106b抑制物組、陰性對照組和空白對照組,且miR-106b抑制物組均低于陰性對照組和空白對照組,說明miR-106b可能參與了CNE-2細胞遷移和侵襲過程,但具體機制尚待進一步研究明確。
綜上所述,miR-106b在鼻咽癌組織中呈高表達,過表達miR-106b可促進鼻咽癌CNE-2細胞增殖、遷移和侵襲能力,其機制可能與促進細胞進入S期有關;提示miR-106b有望成為鼻咽癌基因治療的潛在靶點。
[1] 王盼盼,季明芳,吳標華.鼻咽癌高發區人群患鼻咽癌風險率的動態觀察[J].中華腫瘤防治雜志,2014,21(15):1144-1147.
[2] Kim W, Lee WB, Lee J, et al. Traditional herbal medicine as adjunctive therapy for nasopharyngeal cancer: a systematic review and meta-analysis[J]. Integr Cancer Ther, 2015,14(3):212-220.
[3] Mori M, Triboulet R, Mohseni M, et al. Hippo signaling regulates microprocessor and links cell-density-dependent miRNA biogenesis to cancer[J]. Cell, 2014,156(5):893-906.
[4] 董曦,孫桂波,邢小燕,等.Micro RNA-106b-25簇與腫瘤[J].中國藥理學通報,2014,30(12):1639-1641.
[5] Tan W, Li Y, Lim SG, et al. miR-106b-25/miR-17-92 clusters: polycistrons with oncogenic roles in hepatocellular carcinoma[J]. World J Gastroenterol, 2014,20(20):5962-5972.
[6] Tian Y, Tian Y, Zhang W, et al. Junctional adhesion molecule-A, an epithelial-mesenchymal transition inducer, correlates with metastasis and poor prognosis in human nasopharyngeal cancer[J]. Carcinogenesis, 2015,36(1):41-48.
[7] 申剛,嘉紅云,陳德基,等.miR-106b表達對人肝細胞肝癌細胞增殖能力的影響[J].中華腫瘤雜志,2014,36(7):489-495.
[8] 段繼惠,楊磊,王巍,等.微小RNA-106b靶向下調腺瘤性息肉病基因表達促進Hep-2細胞的增殖[J].中華實驗外科雜志,2015,32(7):1650-1652.
[9] 葉敏華,張安玲,吳雷,等.反義抑制miR-106b表達對人腦膠質瘤細胞增殖和侵襲的影響[J].中華神經外科雜志,2015,31(1):60-65.
[10] Xiang W, He J, Huang C, et al. miR-106b-5p targets tumor suppressor gene SETD2 to inactive its function in clear cell renal cell carcinoma[J]. Oncotarget, 2015,6(6):4066-4079.
[11] Prasad R, Katiyar SK. Down-regulation of miRNA-106b inhibits growth of melanoma cells by promoting G1-phase cell cycle arrest and reactivation of p21/WAF1/Cip1 protein[J]. Oncotarget, 2014,5(21):10636-10649.
[12] Zhang H, Yan X. Cantharidin modulates the E2F1/MCM7-miR-106b-93/p21- PTEN signaling axis in MCF-7 breast cancer cells[J]. Oncol Lett, 2015,10(5):2849-2855.
Expression of miR-106b in nasopharyngeal carcinoma tissues and its effects on the biological characteristics of nasopharyngeal carcinoma CNE-2 cells
ZHOUShixia,WANGHaili,WANGHaoxun
(TheSecondAffiliatedHospitalofZhengzhouUniversity,Zhengzhou450014,China)
ObjectiveTo investigate the expression of miR-106b in nasopharyngeal carcinoma tissues and its effects on the biological characteristics of nasopharyngeal carcinoma CNE-2 cells.MethodsSixty-eight cases of surgical resection specimens from first treatment patients with nasopharyngeal carcinoma and 45 cases of nasopharyngeal biopsy specimens from patients with chronic nasopharyngitis were selected. The expression of miR-106b was detected by using real-time PCR technology. The relationship between the expression of miR-106b and clinicopathological parameters in patients with nasopharyngeal carcinoma was analyzed. The nasopharyngeal carcinoma CNE-2 cells in the logarithmic growth phase were divided into four groups (1×106cells/group). Cells in the miR-106b mimics group and miR-106b inhibitor group were transected with miR-106b mimics and miR-106 inhibitor, respectively. Cells in the negative control group were transfected with negative control sequence, while cells in the blank control group were not treated. The cell proliferation of each transfected group was tested by using MTT assay. The cell cycle of each transfected group was detected by using flow cytometry. The cell migration and invasion of each transfected group were detected by using Transwell experiment.ResultsThe relative expression levels of miR-106b in the nasopharyngeal carcinoma tissues and chronic nasopharyngitis nasopharyngeal tissues were 1.57±0.15 and 1.14±0.12, respectively,P<0.05. The expression of miR-106b in the nasopharyngeal carcinoma tissues was related with lymph node metastasis, carotid sheath violations, and skull base violations (allP<0.05). The relative expression level in each transfection group: the miR-106b mimic group was significantly higher than the miR-106b inhibitor group, the negative control group and blank control group, and the miR-106b inhibitor group was lower than the negative control group and blank control group, allP<0.05. The proliferation of each transfection group: A values after cell inoculation 24, 48, 72 and 96 h in the miR-106b mimic group were higher than those of the miR-106b inhibitor group, negative control group and blank control group, while the miR-106b inhibitor group was lower than the negative control group and blank control group, allP<0.05. The cell cycle of each transfection group: the proportion of S phase in the miR-106b mimics group was higher than that of the miR-106b inhibitor group, the negative control group and blank control group, while the miR-106b inhibitor group was lower than the negative control group and blank control group, allP<0.05. The cell migration and cell invasion in each transfection group: the number of migration cells and invasion cells in the miR-106b mimics group was higher than that of the miR-106b inhibitor group, negative control group and blank control group, and the miR-106b inhibitor group was lower than the negative control group and blank control group, allP<0.05.ConclusionmiR-106b is highly expressed in nasopharyngeal carcinoma tissue, and the miR-106b overexpression may promote proliferation, migration, and invasion of nasopharyngeal carcinoma CNE-2 cells.
nasopharyngeal carcinoma; miR-106b; CNE-2 cells; cell proliferation; cell migration
河南省科技發展計劃(142102310087)。
周士霞(1979-),女,主治醫師,主要研究方向為惡性腫瘤的臨床及基礎研究。E-mail: 1541421042@qq.com
10.3969/j.issn.1002-266X.2017.20.003
R739.6
A
1002-266X(2017)20-0009-04
2016-07-05)

