不同劑量利拉魯肽對(duì)2型糖尿病患者血壓影響的Meta分析
殷云杰,楊 松,陳燕春,季燕妮,黃 凱,嚴(yán)金川
糖尿病是一組以血糖升高為特征的代謝性疾病,以2 型糖尿病較為多見。近年來(lái)隨著人們生活水平提高及人口老齡化進(jìn)程加劇,2型糖尿病發(fā)病率呈逐年上升趨勢(shì),已成為威脅人們生命健康的慢性疾病之一,且糖尿病合并高血壓、冠心病患者預(yù)后不佳[1]。利拉魯肽是胰高血糖素樣肽1(glucagon-like peptide 1,GLP-1)類似物,具有促進(jìn)胰島素合成和分泌、胰島β細(xì)胞增殖及抑制胰高血糖素分泌等作用;此外,其還具有抑制食欲、減輕體質(zhì)量、調(diào)節(jié)脂代謝等作用[2]。近年來(lái),GLP-1類似物或GLP-1受體激動(dòng)劑在心血管領(lǐng)域的應(yīng)用越來(lái)越受關(guān)注[3]。多個(gè)隨機(jī)對(duì)照研究(randomized controlled trails,RCT)結(jié)果顯示,GLP-1類似物利拉魯肽能有效改善胰島素抵抗現(xiàn)象、控制血糖和糖化血紅蛋白,并能降低收縮壓、改善脂代謝及抑制炎性因子釋放等[4-6]。但也有研究結(jié)果顯示,利拉魯肽對(duì)血壓無(wú)明顯影響[7-8]。本研究采用Meta分析方法評(píng)價(jià)不同劑量利拉魯肽對(duì)2型糖尿病患者血壓的影響,旨在為利拉魯肽治療2型糖尿病合并高血壓患者提供循證證據(jù)。
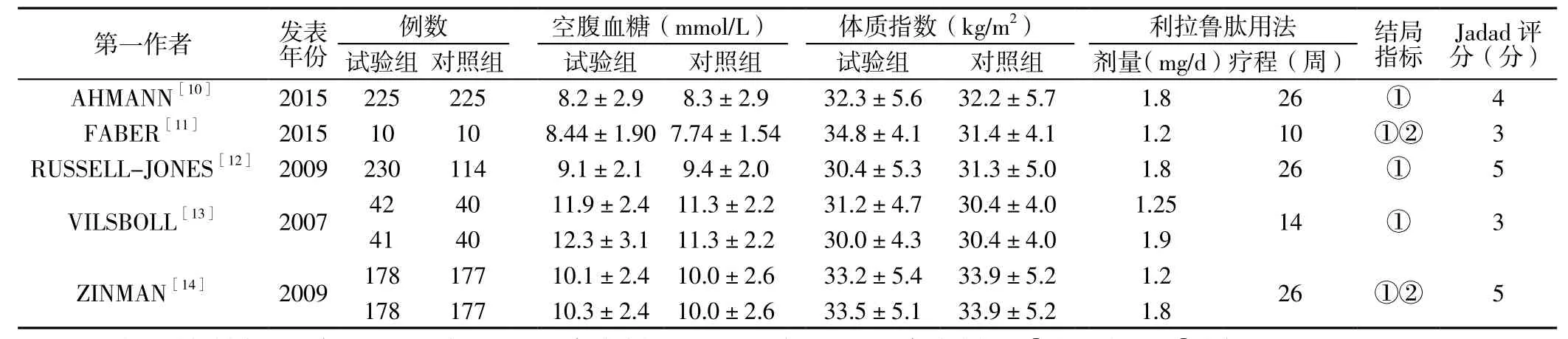
表1 納入文獻(xiàn)的基本特征Table 1 Basic characteristics of the involved literatures
1 資料與方法
1.1 檢索策略 計(jì)算機(jī)檢索PubMed、The Cochrane Library、EMBase、萬(wàn)方數(shù)據(jù)知識(shí)服務(wù)平臺(tái)、中國(guó)知網(wǎng)等數(shù)據(jù)庫(kù),中文檢索詞:“利拉魯肽”“高血壓”“血壓”“2型糖尿病”,英文檢索詞:“l(fā)iraglutide”“hypertension”“blood pressure”“type 2 diabetes mellitus”,檢索時(shí)間從建庫(kù)至2016年4月,由兩名研究者獨(dú)立完成文獻(xiàn)檢索。
1.2 文獻(xiàn)納入與排除標(biāo)準(zhǔn) 文獻(xiàn)納入標(biāo)準(zhǔn):(1)研究類型:RCT。(2)研究對(duì)象:2型糖尿病患者,年齡15~80歲。(3)干預(yù)措施:試驗(yàn)組患者采用利拉魯肽治療,并根據(jù)利拉魯肽劑量分為小劑量組(利拉魯肽1.2 mg/d)和大劑量組(利拉魯肽1.8 mg/d);對(duì)照組患者給予安慰劑。對(duì)照組、試驗(yàn)組患者治療時(shí)間≥8周。(4)結(jié)局指標(biāo):收縮壓和舒張壓。文獻(xiàn)排除標(biāo)準(zhǔn):(1)綜述;(2)重復(fù)文獻(xiàn)。
1.3 資料提取 由兩名研究者獨(dú)立提取、評(píng)價(jià)資料,如遇分歧則重新閱讀原文查找證據(jù)或咨詢第三方解決。提取內(nèi)容包括第一作者、發(fā)表年份、例數(shù)、空腹血糖、體質(zhì)指數(shù)、利拉魯肽用法、結(jié)局指標(biāo)。
1.4 質(zhì)量評(píng)價(jià)標(biāo)準(zhǔn) 采用Jadad評(píng)分法[9]評(píng)價(jià)納入文獻(xiàn)質(zhì)量,包括隨機(jī)化、盲法及失訪與退出3個(gè)方面。隨機(jī)化評(píng)分標(biāo)準(zhǔn):采用計(jì)算機(jī)產(chǎn)生隨機(jī)數(shù)字或其他類似方法記為2分,屬于隨機(jī)試驗(yàn)但未描述隨機(jī)方法記為1分,采用交替分配方法記為0分;盲法評(píng)分標(biāo)準(zhǔn):采用完全一致的安慰劑或類似方法記為2分,試驗(yàn)陳述為盲法但未具體描述記為1分,未采用盲法或盲法不恰當(dāng)記為0分;失訪與退出評(píng)分標(biāo)準(zhǔn):描述失訪與退出例數(shù)及原因記為1分,未描述失訪與退出例數(shù)及原因記為0分。Jadad評(píng)分≥3分判定為文獻(xiàn)質(zhì)量較高。
1.5 統(tǒng)計(jì)學(xué)方法 應(yīng)用 RevMan 5.3軟件進(jìn)行 Meta分析,采用Stata 12.0軟件進(jìn)行數(shù)據(jù)分析,連續(xù)變量以SD及其95%CI表示,統(tǒng)計(jì)學(xué)異質(zhì)性檢驗(yàn)采用χ2檢驗(yàn),P≥0.05且I2≤50%表明各文獻(xiàn)間無(wú)統(tǒng)計(jì)學(xué)異質(zhì)性,采用固定效應(yīng)模型進(jìn)行Meta分析;P<0.05且I2>50%表明各文獻(xiàn)間有統(tǒng)計(jì)學(xué)異質(zhì)性,采用隨機(jī)效應(yīng)模型進(jìn)行Meta分析;文獻(xiàn)發(fā)表偏倚分析采用Begg's檢驗(yàn)。以P<0.05為差異有統(tǒng)計(jì)學(xué)意義。
2 結(jié)果
2.1 檢索結(jié)果 初步檢索到相關(guān)文獻(xiàn)366篇,閱讀摘要排除重復(fù)文獻(xiàn)及不符合納入標(biāo)準(zhǔn)文獻(xiàn)357篇,進(jìn)一步閱讀全文排除2篇非RCT、2篇2型糖尿病診斷不準(zhǔn)確文獻(xiàn),最終納入5篇文獻(xiàn)[10-14],共包括1 470例患者,其中文獻(xiàn)[13-14]同時(shí)設(shè)置了小劑量組和大劑量組,納入文獻(xiàn)的基本特征詳見表1。
2.2 Meta 分析結(jié)果
2.2.1 小劑量利拉魯肽對(duì)血壓的影響
2.2.1.1 收縮壓 3 篇文獻(xiàn)[11,13-14]報(bào)道了治療后收縮壓,各文獻(xiàn)間無(wú)統(tǒng)計(jì)學(xué)異質(zhì)性(P=0.65,I2=0%),采用固定效應(yīng)模型進(jìn)行Meta分析;結(jié)果顯示,治療后小劑量組患者收縮壓低于對(duì)照組,差異有統(tǒng)計(jì)學(xué)意義〔MD=-5.82,95%CI(-8.31,-3.32),P<0.000 01,見圖 1〕。
2.2.1.2 舒張壓 2 篇文獻(xiàn)[11,14]報(bào)道了治療后舒張壓,各文獻(xiàn)間無(wú)統(tǒng)計(jì)學(xué)異質(zhì)性(P=0.89,I2=0%),采用固定效應(yīng)模型進(jìn)行Meta分析;結(jié)果顯示,治療后小劑量組和對(duì)照組患者舒張壓比較,差異無(wú)統(tǒng)計(jì)學(xué)意義〔MD=-1.54,95%CI(-3.40,0.32),P=0.10,見圖 2〕。
2.2.2 大劑量利拉魯肽對(duì)血壓的影響
2.2.2.1 收縮壓 4 篇文獻(xiàn)[10,12-14]報(bào)道了治療后收縮壓,各文獻(xiàn)間無(wú)統(tǒng)計(jì)學(xué)異質(zhì)性(P=0.28,I2=21%),采用固定效應(yīng)模型進(jìn)行Meta分析;結(jié)果顯示,治療后大劑量組患者收縮壓低于對(duì)照組,差異有統(tǒng)計(jì)學(xué)意義〔MD=-4.45,95%CI(-5.59,-2.94),P<0.000 01,見圖 3〕。
2.2.2.2 舒張壓 2 篇文獻(xiàn)[10,14]報(bào)道了治療后舒張壓,各文獻(xiàn)間無(wú)統(tǒng)計(jì)學(xué)異質(zhì)性(P=0.73,I2=0%),采用固定效應(yīng)模型進(jìn)行Meta分析;結(jié)果顯示,治療后大劑量組與對(duì)照組患者舒張壓比較,差異無(wú)統(tǒng)計(jì)學(xué)意義〔MD=-0.84,95%CI(-2.07,0.39),P=0.18,見圖 4〕。
2.2.3 發(fā)表偏移 Begg's檢驗(yàn)結(jié)果顯示,報(bào)道收縮壓的文獻(xiàn)無(wú)發(fā)表偏倚(t值分別為-2.80、-1.14,P值分別為0.22、0.37,見圖5~6)。
3 討論
近年來(lái),糖尿病發(fā)病率呈逐年上升趨勢(shì),其并發(fā)癥較為嚴(yán)重,已成為新的公共衛(wèi)生問題之一。流行病學(xué)調(diào)查結(jié)果顯示,高血壓是2型糖尿病患者的嚴(yán)重并發(fā)癥之一,約75.0%的2型糖尿病患者合并高血壓,且糖尿病合并高血壓患者預(yù)后較差[15]。因此,控制血壓對(duì)改善糖尿病患者預(yù)后具有重要意義。
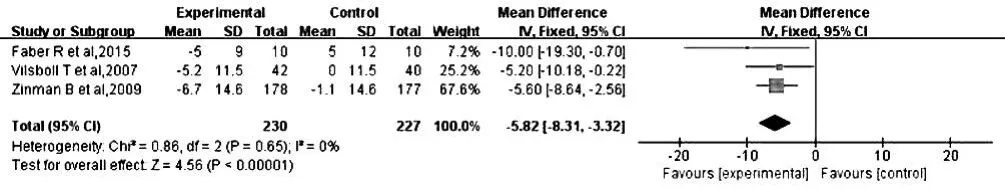
圖1 對(duì)照組和小劑量組患者治療后收縮壓比較森林圖Figure 1 Forest plot for comparison of SBP between control group and low-dose group after treatment
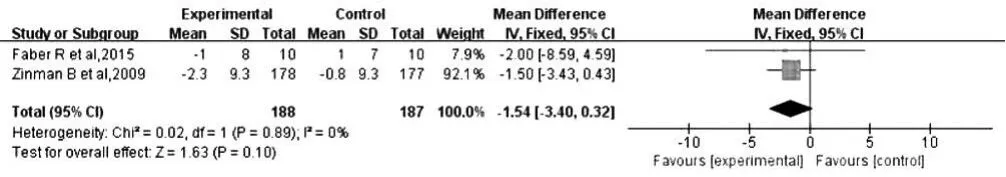
圖2 對(duì)照組和小劑量組患者治療后舒張壓比較森林圖Figure 2 Forest plot for comparison of DBP between control group and low-dose group after treatment
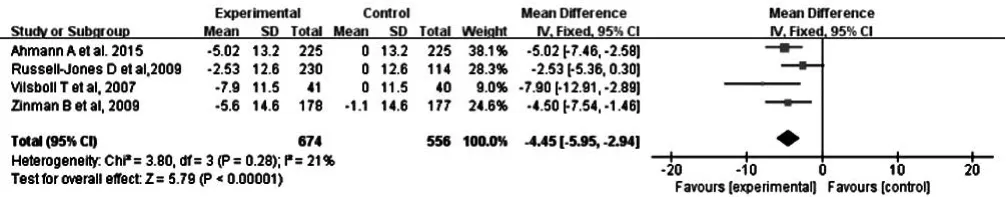
圖3 對(duì)照組和大劑量組患者治療后收縮壓比較森林圖Figure 3 Forest plot for comparison of SBP between control group and high-dose group after treatment
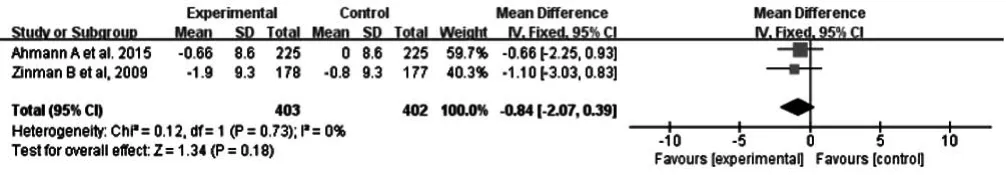
圖4 對(duì)照組和大劑量組患者治療后舒張壓比較森林圖Figure 4 Forest plot for comparison of DBP between control group and high-dose group after treatment

圖5 報(bào)道小劑量利拉魯肽治療后收縮壓文獻(xiàn)發(fā)表偏倚的漏斗圖Figure 5 Funnel plot for publication bias of involved literatures reported SBP after treatment of low-dose liraglutide
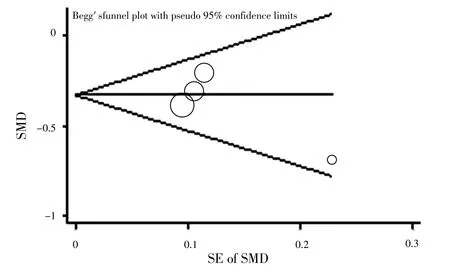
圖6 報(bào)道大劑量利拉魯肽治療后收縮壓文獻(xiàn)發(fā)表偏倚的漏斗圖Figure 6 Funnel plot for publication bias of involved literatures reported SBP after treatment of high-dose liraglutide
GLP-1類似物利拉魯肽或GLP-1受體激動(dòng)劑艾塞那肽和二肽基肽酶4(DPP-Ⅳ)抑制劑西格列汀、維格列汀是作用于腸促胰島素的主要藥物[16]。GLP-1由小腸L細(xì)胞合成,空腹?fàn)顟B(tài)下血漿GLP-1水平較低,進(jìn)食后其水平迅速升高,其主要通過G蛋白耦聯(lián)受體發(fā)揮作用。既往研究表明,GLP-1受體主要表達(dá)于胰島β細(xì)胞、心臟和血管等[17],被激活后可導(dǎo)致腺苷酸環(huán)化酶活化,進(jìn)而增加細(xì)胞內(nèi)環(huán)磷酸腺苷(cAMP)濃度及激活蛋白激酶A(PKA)通路,故GLP-1能直接通過cAMP-PKA通路刺激胰島素分泌[18]。利拉魯肽是臨床常用的GLP-1類似物,是GLP-1與脂肪酸的結(jié)合體,其通過與清蛋白結(jié)合而抑制DPP-Ⅳ分解,且腎臟排泄率較低[19]。
現(xiàn)有研究結(jié)果顯示,利拉魯肽能有效降低2型糖尿病患者血糖、糖化血紅蛋白及體質(zhì)指數(shù)等[20];此外,其還能降低收縮壓及抑制炎性因子分泌等[21],但對(duì)舒張壓無(wú)明顯影響[22]。目前,利拉魯肽的降壓機(jī)制尚未完全闡明。動(dòng)物實(shí)驗(yàn)結(jié)果顯示,利拉魯肽對(duì)鹽敏感大鼠具有抗高血壓、保護(hù)心肌和腎臟等作用,分析其作用機(jī)制主要是利拉魯肽可通過抑制近端腎小管Na+重吸收或減輕腎細(xì)胞中血管緊張素Ⅱ誘導(dǎo)的細(xì)胞外信號(hào)調(diào)節(jié)激酶1/2(ERK1/2)磷酸化而發(fā)揮利鈉、利尿作用[23]。LOVSHIN等[24]研究結(jié)果顯示,利拉魯肽3.0 mg/d對(duì)非2型糖尿病患者收縮壓及舒張壓均無(wú)明顯影響。
本Meta分析結(jié)果顯示,治療后小劑量組和大劑量組患者收縮壓低于對(duì)照組,小劑量組、大劑量組與對(duì)照組患者舒張壓間無(wú)差異,提示利拉魯肽1.2 mg/d或1.8 mg/d均能有效降低2型糖尿病患者收縮壓,但對(duì)舒張壓無(wú)影響。本Meta分析仍存在一定局限性:(1)納入文獻(xiàn)數(shù)量較少,樣本量較小,可能影響研究結(jié)果;(2)納入文獻(xiàn)質(zhì)量不高,可能存在一定程度偏倚。
基于現(xiàn)有文獻(xiàn)證據(jù),利拉魯肽1.2 mg/d或1.8 mg/d均能有效降低2型糖尿病患者收縮壓,但對(duì)舒張壓無(wú)明顯影響。
參考文獻(xiàn)
[1]楊文英.重視糖尿病患者的綜合心血管危險(xiǎn)[J].中華心血管病雜志,2007,35(12):1164-1166.DOI:10.3760/j.issn:0253-3758.2007.12.023.
[2]PRASAD-REDDY L,ISAACS D.A clinical review of GLP-1 receptor agonists:efficacy and safety in diabetes and beyond[J].Drugs Context,2015,4:212283.DOI:10.7573/dic.212283.
[3]RIGATO M,F(xiàn)ADINI G P.Comparative effectiveness of liraglutide in the treatment of type 2 diabetes[J].Diabetes Metab Syndr Obes,2014,7:107-120.DOI:10.2147/DMSO.S37644.
[4]GIGLIO R V,PATTI A M,NIKOLIC D,et al.The extra-glycemic effects of liraglutide:focus on cardiometabolic markers[J].G Ital Cardiol(Rome),2016,17(4):253-258.DOI:10.1714/2214.23896.
[5]KALRA S,BARUAH M P,SAHAY R K,et al.Glucagon-like peptide-1 receptor agonists in the treatment of type 2 diabetes:Past,present,and future[J].Indian J Endocrinol Metab,2016,20(2):254-267.DOI:10.4103/2230-8210.176351.
[6]LUTZ T A,OSTO E.Glucagon-like peptide-1,glucagon-like peptide-2,and lipid metabolism[J].Curr Opin Lipidol,2016,27(3):257-263.DOI:10.1097/MOL.0000000000000293.
[7]LOVSHIN J A,BARNIE A,DEALMEIDA A,et al.Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes[J].Diabetes Care,2015,38(1):132-139.DOI:10.2337/dc14-1958.
[8]GILL A,HOOGWERF B J,BURGER J,et al.Effect of exenatide on heart rate and blood pressure in subjects with type 2 diabetes mellitus:a doubleblind,placebo-controlled,randomised pilot study[J].Cardiovasc Diabetol,2010,9:6.DOI:10.1186/1475-2840-9-6.
[9]MOHER D,JADAD A R,TUGWELL P.Assessing the quality of randomized controlled trials.Current issues and future directions[J].Int J Technol Assess Health Care,1996,12(2):195-208.
[10]AHMANN A,RODBARD H W,ROSENSTOCK J,et al.Efficacy and safety of liraglutide versus placebo added to basal insulin analogues (with or without metformin) in patients with type 2 diabetes:a randomized,placebo-controlled trial[J].Diabetes Obes Metab,2015,17(11):1056-1064.DOI:10.1111/dom.12539.
[11]FABER R,ZANDER M,PENA A,et al.Effect of the glucagonlike peptide-1 analogue liraglutide on coronary microvascular function in patients with type 2 diabetes-a randomized,singleblinded,cross-over pilot study[J].Cardiovasc Diabetol,2015,14:41.DOI:10.1186/s12933-015-0206-3.
[12]RUSSELL-JONES D,VAAG A,SCHMITZ O,et al.Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU):a randomised controlled trial[J].Diabetologia,2009,52(10):2046-2055.DOI:10.1007/s00125-009-1472-y.
[13]VILSBOLL T,ZDRAVKOVIC M,LE-THI T,et al.Liraglutide,a long-acting human glucagon-like peptide-1 analog,given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes[J].Diabetes Care,2007,30(6):1608-1610.
[14]ZINMAN B,GERICH J,BUSE J B,et al.Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes(LEAD-4 Met+TZD)[J].Diabetes Care,2009,32(7):1224-1230.DOI:10.2337/dc08-2124.
[15]WANG B,ZHONG J,LIN H,et al.Blood pressure-lowering effects of GLP-1 receptor agonists exenatide and liraglutide:a meta-analysis of clinical trials[J].Diabetes Obes Metab,2013,15(8):737-749.DOI:10.1111/dom.12085.
[16]NAUCK M.Incretin therapies:highlighting common features and differences in the modes of action of glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors[J].Diabetes Obes Metab,2016,18(3):203-216.DOI:10.1111/dom.12591.
[17]VILSB?LL T.The effects of glucagon-like peptide-1 on the beta cell[J].Diabetes Obes Metab,2009,11(suppl 3):11-18.DOI:10.1111/j.1463-1326.2009.01073.x.
[18]CHON S,GAUTIER J F.An Update on the Effect of Incretin-Based Therapies on β-Cell Function and Mass[J].Diabetes Metab J,2016,40(2):99-114.DOI:10.4093/dmj.2016.40.2.99.
[19]SCHEEN A J.Cardiovascular effects of dipeptidyl peptidase-4 inhibitors:from risk factors to clinical outcomes[J].Postgrad Med,2013,125(3):7-20.DOI:10.3810/pgm.2013.05.2659.
[20]張偉,馬維青.胰高糖素樣肽1類似物對(duì)2型糖尿病患者體質(zhì)量的影響及作用機(jī)制研究進(jìn)展[J].山東醫(yī)藥,2015,55(31):92-95. DOI:10.3969/j.issn.1002-266X.2015.31.038
[21]BLONDE L,PENCEK R,MACCONELL L.Association among weight change,glycemic control,and markers of cardiovascular risk with exenatide once weekly:a pooled analysis of patients with type 2 diabetes[J].Cardiovasc Diabetol,2015,14:12.DOI:10.1186/s12933-014-0171-2.
[22]SCOTT L J.Liraglutide:a review of its use in adult patients with type 2 diabetes mellitus[J].Drugs,2014,74(18):2161-2174.DOI:10.1007/s40265-014-0321-6.
[23]MORENO C,MISTRY M,ROMAN R J.Renal effects of glucagonlike peptide in rats[J].Eur J Pharmacol,2002,434(3):163-167.
[24]LOVSHIN J A,BARNIE A,DEALMEIDA A,et al.Liraglutide promotes natriuresis but does not increase circulating levels of atrial natriuretic peptide in hypertensive subjects with type 2 diabetes[J].Diabetes Care,2015,38(1):132-139.DOI:10.2337/dc14-1958.
- 實(shí)用心腦肺血管病雜志的其它文章
- 子癇并顱內(nèi)靜脈竇血栓形成、腦出血及可逆性后部腦病綜合征一例報(bào)道
- 以暈厥為首發(fā)癥狀的鼻咽癌一例報(bào)道并文獻(xiàn)復(fù)習(xí)
- 依達(dá)拉奉聯(lián)合丁苯酞對(duì)急性腦梗死患者的影響研究
- 曲美他嗪聯(lián)合運(yùn)動(dòng)康復(fù)對(duì)慢性心力衰竭患者心腎功能及甲狀旁腺素的影響
- 硝普鈉和多巴胺聯(lián)合凍干重組人腦利鈉肽對(duì)難治性心力衰竭患者的影響
- 硝苯地平對(duì)經(jīng)橈動(dòng)脈入路行冠狀動(dòng)脈造影術(shù)/經(jīng)皮冠狀動(dòng)脈介入術(shù)的冠心病患者內(nèi)皮依賴性血管舒張功能的影響

