快速老化小鼠模型NgR、磷酸化Tau蛋白表達情況及其關系的研究
溫世榮 許杰 趙延峰 鄭姣琳 李鶴 趙秀麗 張欣
[摘要] 目的 利用Nogo受體(NgR)拮抗劑NEP1-40研究P8品系快速老化小鼠(SAMP8)鼠腦中NgR與磷酸化Tau蛋白(p-Tau)表達情況,探討NgR可能作用。 方法 6月齡抗快速老化系1(SAMR1)雄性小鼠為對照組(A組),3月齡SAMP8鼠(B組),6月齡SAMP8鼠為假治療組(C組)和治療組(N組),每組6只。C組小鼠腹腔注射0.9%生理鹽水0.2 mL、N組小鼠0.2 mL NEP1-40各14 d。治療前后分別采用Morris水迷宮試驗監測小鼠行為學改變,利用免疫組化染色檢測神經細胞內p-Tau和NgR表達的變化。 結果 B、C、N組與A組比較,水迷宮試驗潛伏期明顯延長、穿越平臺次數明顯減少(P < 0.05);N組第二次與第一次水迷宮實驗比較,潛伏期明顯縮短、跨越平臺次數明顯增多(P < 0.05)。神經元胞漿、突起NgR陽性棕黃色顆粒狀結構與細胞膜關系密切,C組海馬NgR陽性細胞計數和光密度值較A組和N組明顯增多(P < 0.05)。C組p-Tau陽性細胞沉積較A組和N組明顯增加(P < 0.05)。 結論 SAMP8鼠隨月齡增大,學習記憶能力減退,NgR及p-Tau在海馬沉積增多明顯,與小鼠老化程度相一致。NgR拮抗劑NEP1-40可能是通過下調NgR表達、減少下游p-Tau表達量,改善小鼠學習及記憶能力。
[關鍵詞] 阿爾茨海默病;Tau蛋白;Nogo受體;NEP1-40
[中圖分類號] R749.16? ? ? ? ? [文獻標識碼] A? ? ? ? ? [文章編號] 1673-7210(2019)09(b)-0022-04
The expression and relationship between NgR and p-Tau in brains of senescence accelerated mice
WEN Shirong1? ?XU Jie2? ?ZHAO Yanfeng3? ?ZHENG Jiaolin3? ?LI He1? ?ZHAO Xiuli1? ?ZHANG Xin1
1.Department of Neurology, the First Affiliated Hospital of Harbin Medical University, Heilongjiang Province, Harbin 150001, China; 2.Department of Neurology, the Second Hospital of Qinhuangdao, Hebei Province, Qinhuangdao? ?066600, China; 3.Department of Neurology, the Second Affiliated Hospital of Harbin Medical University, Heilongjiang Province, Harbin? ?150086, China
[Objective] To investigate the expression of NgR and p-tau in senescence accelerated mouse P8 (SAMP8) was studied by using Nogo receptor (NgR) antagonist NEP1-40, explore the possible role of NgR. Methods Six months old senescence accelerated mouse-resistence 1 (SAMR1) male mice were the control group (group A); 3 months old SAMP8 mice (group B). Sham treatment group (group C) and treatment group (group N) of SAMP8 mice at 6 months of age, with 6 mice in each group. Mice in group C were intraperitoneally injected with 0.2 mL 0.9% normal saline, and mice in group N were intraperitoneally injected with 0.2 mL NEP1-40 for 14 days. Morris water maze test was used to monitor behavioral changes in mice before and after treatment, and immunohistochemical staining was used to detect changes in p-Tau and NgR expression in nerve cells. Results Compared with group A, the incubation periods of water maze test in group B, C and N were significantly prolonged, and the times of crossing platform were significantly reduced (P < 0.05). Compared with the first water maze experiment, the incubation period of group N was significantly shortened and the times of crossing platform were significantly increased (P < 0.05). The NgR positive cell count and optical density of the hippocampus in group C were significantly increased compared with those in group A and group N (P < 0.05). The deposition of p-Tau positive cells in group C was significantly reduced compared with that in group A and group N(P < 0.05). Conclusion The learning and memory ability of SAMP8 mice decreased with the aging of the month, and NgR and p-Tau increased significantly in the hippocampus, which was consistent with the aging degree of the mice. NgR antagonist NEP1-40 may improve the learning and memory ability of mice by down-regulating NgR expression and reducing downstream p-Tau expression.
[Key words] Alzheimer′s disease; Tau protein; NgR; NEP1-40
阿爾茨海默病(Alzheimer′s disease,AD)患者海馬錐體層細胞Nogo受體(NgR)免疫活性高達50%以上,特別是CA1~CA2區有大量依賴磷酸化Tau蛋白單克隆抗體AT-8免疫陽性細胞,綁定和識別磷酸化Tau蛋白絲氨酸199/202位點[1]。NgR陽性神經元與神經纖維纏結樣改變及AT-8在AD患者海馬CA1區共區域化,提示NgR可能與AD神經纖維纏結相關。但AD者CA1區約2/3NgR免疫活性神經元AT-8陰性[2]。本研究利用快速老化小鼠模型,腹腔注射NgR抑制劑NEP1-40,檢測NgR表達情況及對Tau蛋白表達影響,以闡釋NgR在AD病理改變中的作用。
1 材料與方法
1.1 實驗動物
3、6月齡雄性P8品系快速老化小鼠(SAMP8),6月齡雄性抗快速老化系1(SAMR1)鼠,購自天津中醫藥大學第一附屬醫院(合格證書:0004580),實驗程序由哈爾濱醫科大學附屬一院動物使用委員會批準,動物倫理號:2016010,置于SPF環境飼養和護理。
1.2 儀器與試劑
1.2.1 儀器? 小鼠Morris水迷宮儀、自動數據采集及處理系統(黑龍江省中醫藥大學);尼康(Nikon)50i研究型正置顯微鏡;NIS-ElementsF 3.21圖像采集軟件(Nikon Inc. Japan);圖像分析軟件(ImagePro-Plus6,MediaCybernetics,Silver Spring,MD,USA)。
1.2.2 試劑? Rabbit Anti-Nogo receptor、兔二抗(博士德生物),p-Tau(sc-101813,SANTA CRUZ生物),NEP1-40(BOC Sciences,475221-20-6)等。
1.3 實驗方法
1.3.1 實驗動物分組? 小鼠適應性飼養1周分6月齡SAMR1組(A組)、3月齡SAMP8組(B組),6月齡SAMP8小鼠隨機分為假治療組(生理鹽水,C組)、治療組(NEP1-40,N組)。
1.3.2 水迷宮實驗(Morris水迷宮)? 第1、2天訓練(可視平臺),第3~5天定向航行(隱蔽平臺),第6天空間探索(去平臺),圖像采集分析系統記錄動物游泳軌跡數據。定位航行隨機取東、西、南、北4個起始位置,找到水下平臺時間(s)即潛伏期;空間搜索撤除平臺,動物由原象限對側入水,記錄動物進入目標象限次數。
1.3.3 藥物干預及二次水迷宮實驗? 初次水迷宮后,C組腹腔注射0.9%生理鹽水0.2 mL/只,N組12.5 μg/(kg·d)NEP1-40(0.2 mL/只),每日1次,連續2周。A組和B組不干預。按初次方法重復水迷宮實驗并采集數據。
1.3.4 灌注取腦及切片? 二次水迷宮后,所有小鼠用生理鹽水60~100 mL、4%多聚甲醛100~150 mL左心室灌注,取腦,4%甲醛固定、常規梯度酒精脫水、二甲苯透明,石蠟包埋,3 μm連續冠狀切片。
1.3.5 免疫組織化學染色? 石蠟切片脫蠟,脫水,熱抗原修復、室溫兔血清封閉。一抗p-Tau(1∶80)、NgR(1∶20) 4°C過夜。兔二抗,室溫孵育20 min。DAB染色。蘇木精復染,脫水、透明、封片。每標本取5張切片,高倍鏡(400×)海馬區不重疊5個視野觀察拍照,陽性細胞(神經元胞漿、突起接近細胞膜棕黃色顆粒狀結構為陽性)計數;image pro-plus 5.0圖像采集軟件采樣、圖像分析系統選取陽性細胞測定其積分光密度(IOD值)。
1.4 統計學方法
采用SPSS 17.0對所得數據進行統計學分析,計量資料采用均數±標準差(x±s)表示,多樣本均數比較用方差分析;組間比較采用t檢驗;不符合正態分布采用秩和檢驗。以P < 0.05為差異有統計學意義。
2 結果
2.1 Morris水迷宮
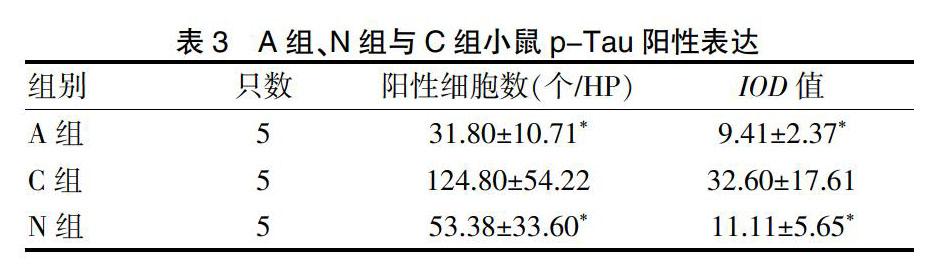
A、B、C三組第二次潛伏期、穿越平臺次數與第一次比較,差異無統計學意義(P < 0.05)。N組第二次潛伏期短于第一次、穿越平臺次數多于第一次;C組第二次潛伏期明顯長于A、B組,穿越平臺次數少于A、B組,N組第二次潛伏期短于C組,穿越平臺次數多于C組,差異均有統計學意義(均P < 0.05)。表1。
表1? ?各組動物水迷宮實驗結果(x±s)
注:與本組第一次比較,*P < 0.05;與A組同期比較,△P < 0.05;與B組同期比較,◇P < 0.05;與C組同期比較,&P < 0.05
2.2 免疫組化結果
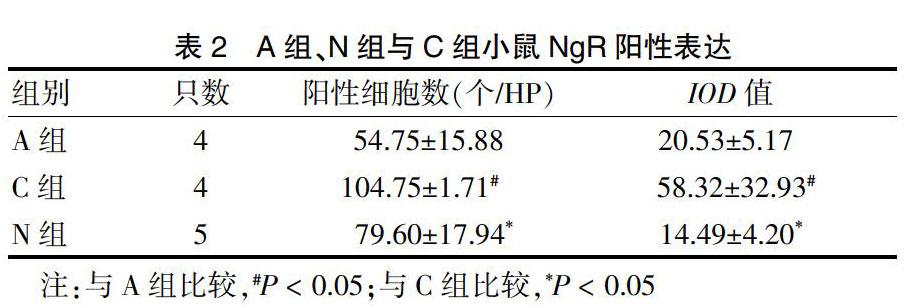
2.2.1 A組、N組與C組小鼠NgR表達情況? C組陽性細胞數和IOD值明顯高于A組和N組(P < 0.05)。見表2、圖1(封四)。
表2? ?A組、N組與C組小鼠NgR陽性表達
注:與A組比較,#P < 0.05;與C組比較,*P < 0.05
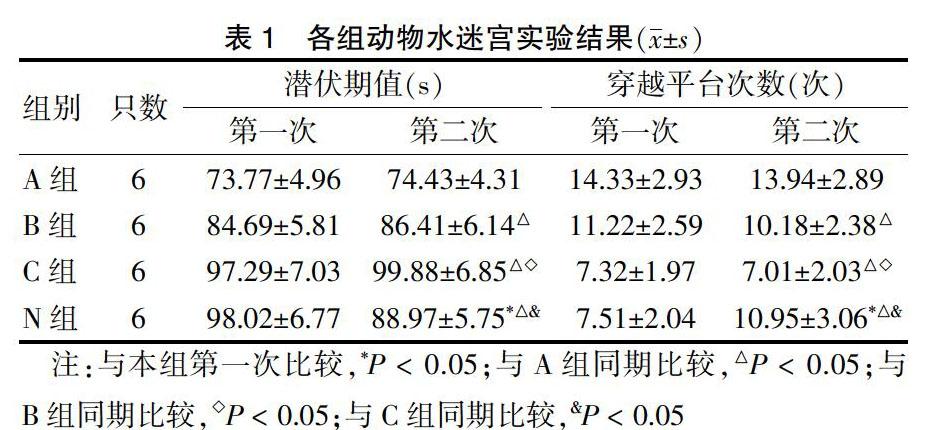
2.2.2 A組、N組與C組小鼠p-Tau表達情況? C組較A組和B組海馬陽性細胞數和IOD值明顯高(P < 0.05)。見表3、圖2(封四)。
表3? ?A組、N組與C組小鼠p-Tau陽性表達
3 討論
AD是最常見的老年癡呆,主要病理特征是神經細胞外β淀粉樣沉積和神經元細胞內神經元纖維纏結,致神經突觸和線粒體結構/功能異常及神經元丟失[3-5]。
水迷宮實驗是通過評價空間學習和記憶能力從而進行認知疾病模型驗證和治療評估[6]。SAMP8 2月齡就開始出現學習記憶功能衰退,呈增齡性加速衰退,SAMR1無此類變化[7-8]。本研究結果顯示,SAMP8小鼠與同月齡SAMR1比較的確出現空間學習記憶功能障礙,注射NgR抑制劑NEP1-40后,小鼠學習及記憶能力有所改善。
NgR作為髓鞘相關蛋白共同受體,其信號通路限制神經元可塑性,抑制損傷后軸突再生,調節β淀粉樣蛋白代謝,可能參與其病理過程[9-10]。另有研究[11]顯示,皮質及海馬等區域NgR可維持神經元回路穩定性及結構可塑性,促進突觸聯絡和長時程記憶。已有研究[12-13]顯示有目的地減少NgR表達,腦內β淀粉樣蛋白沉積和營養不良神經炎癥細胞增多,提示NgR可能有神經保護作用。本研究中,NgR免疫活性細胞主要分布在海馬角和海馬齒狀回,與以往研究NgR分布區一致。快速老化小鼠大腦海馬NgR陽性神經元總數明顯高于非老化模型,支持NgR在海馬區有促進AD病理過程的作用。
NEP1-40與NgR氨基端40個氨基酸殘基結構相同,和NgR下游信號分子競爭性結合,不激活NgR信號通路,一方面阻斷Rho信號傳導途徑,另一方面阻止Nogo-66與NgR結合后介導的生長錐潰變作用,恢復受損中樞神經系統軸突生長和功能,促進神經功能再生。尚可延長NgR低水平表達時程,下調NgR回升時效,進一步發揮神經保護作用[14-15]。本研究中,N組小鼠注射NEP1-40后,水迷宮實驗結果明顯好于非藥物干預組,提示NEP1-40確有保護作用。免疫組化顯示N組NgR免疫陽性細胞數量明顯減少,提示NEP1-40通過競爭性與NgR底物結合,使NgR活性降低或喪失、表達下調,改變NgR神經抑制作用。
Tau促進微管蛋白聚合形成微管并維持穩定性,異常磷酸化不能有效結合并穩定微管,致神經元退化[16]。AD患者異常磷酸化Tau蛋白導致神經纖維纏結和纖維網形成,神經元死亡后形成細胞外纏結,其數量及定位與認知功能下降相關,在AD認知功能缺陷中發揮著關鍵作用[17-19]。有研究顯示,NgR陽性神經元細胞出現神經纖維纏結樣改變和大量NgR陽性神經元與p-Tau蛋白在海馬CA1區共表達,提示NgR可能與AD的神經纖維纏結相關[20]。NgR側鏈含有富含亮氨酸的重復序列,調節蛋白間相互作用。NgR特異生物物理學屬性可能使NgR更容易綁定到其他蛋白質[9]。NgR除了以一種未被確定的方式抑制神經軸突生長外,可能還有其他未被發現的功能。小鼠腦中發現NgR配體Nogo-A與α微管蛋白共免疫沉淀,可能與微管穩定性有關。據此推測NgR可能參與Tau蛋白過度磷酸化,與神經骨架相關聯并參與AD的神經纖維纏結[21-22]。本研究發現,快速老化小鼠海馬可見大量p-Tau蛋白沉積,N組較C組p-Tau蛋白陽性細胞明顯減少,與應用NgR抑制劑后NgR表達減少一致,提示NgR可能作為一個上游蛋白直接影響異常p-Tau表達或是發揮作用。
本研究結果顯示,在AD病理改變及病情進展方面,異常p-Tau蛋白是一個關鍵因素,NgR可能通過對該蛋白表達的影響發揮作用,可以考慮將NgR作為AD治療靶點進一步深入研究。
[參考文獻]
[1]? Zhu HY,Guo HF,Hou HL,et al. Incresed expression of the Nogo receptor in the hippocampus and its relation to the neuropathology in Alzheimer′s disease [J]. Hum Pathol,2007,38(3):426-434.
[2]? ActBraak H,Zetterberg H,Del TK,et al. Intraneuronal tau aggregation precedes diffuse plaque deposition,but amyloid-β changes occur before increases of tau in cerebrospinal fluid [J]. Acta Neuropathol,2013,126(5):631-641.
[3]? Dietrich K,Bouter Y,Muller M,et al. Synaptic alterations in mouse models for Alzheimer disease-a special focus on N-truncated abeta 4-42 [J]. Molecules,2018,23(4):718-732.
[4]? Zeng Y,Zhang J,Zhu Y,et al. Tripchlorolide improves cognitive deficits by reducing amyloid β and upregulating synapse-related proteins in a transgenic model of Alzheimer's Disease [J]. J Neurochem,2015,133(1):38-52.
[5]? Furotani K,Kamimura K,Yajima T,et al. Suppression of the synaptic localization of a subset of proteins including APP partially ameliorates phenotypes of the Drosophila Alzheimer's disease model [J]. PLoS One,2018,13(9):1-17.
[6]? Bromley-Brits K,Deng Y,Song W. Morris water maze test for learning and memory deficits in Alzheimer's disease model mice [J]. J Vis Exp,2011,20(53):2920-2924.
[7]? Susan AF,Elizabeth R,Michael L,et al. Metformin improves learning and memory in the SAMP8 mouse model of Alzheimer′s disease [J]. J Alzheimer Dis,2019,68(4):1699-1710.
[8]? Lam V,Takechi R,Albrecht MA,et al. Longitudinal Performance of Senescence Accelerated Mouse Prone-Strain 8 (SAMP8) Mice in an Olfactory-Visual Water Maze Challenge [J]. Front Behav Neurosci,2018,12:174-181.
[9]? Barton WA,Liu BP,Tzvetkova D,et al. Structure and axon outgrowth inhibitor binding of the Nogo-66 receptor and related proteins[J]. EMBO J,2003,22(13):3291-3302.
[10]? Ziebell JM,Ray-Jones H,Lifshitz J. Nogo presence is inversely associated with shifts in cortical microglial morphology following experimental diffuse brain injury [J]. Neuroscience,2017,359:209-223.
[11]? Thomas RA,Gibon J,Chen CXQ,et al. The nogo receptor ligand LGI1 regulates synapse number and synaptic activity in hippocampal and cortical neurons [J]. eNeuro,2018,5(4):1-15.
[12]? Fang Y,Wang J,Yao L,et al. The adhesion and migration of microglia to β-amyloid (Aβ) is decreased with aging and inhibited by Nogo/NgR pathway [J]. J Neuroinflammation,2018,15(1):210-225.
[13]? Fang Y,Yao L,Li C,et al. The blockage of the Nogo/NgR signal pathway in microglia alleviates the formation of Aβ plaques and tau phosphorylation in APP/PS1 transgenic mice [J]. J Neuroinflammation,2016,13(1):56-72.
[14]? Xu J,He J,He H,et al. Comparison of RNAi NgR and NEP1-40 in Acting on Axonal Regeneration After Spinal Cord Injury in Rat Models [J]. Mol Neurobiol,2017,54(10):8321-8331.
[15]? Cao Y,Shumsky JS,Sabol MA,et al. Nogo-66 receptor antagonist peptide (NEP1-40) administration promotes functional recovery and axonal growth after lateral funiculus injury in the adult rat [J]. Neurorehabil Neural Repair,2008,22(3):262-278.
[16]? Obulesu M,Venu R,Somashekhar R. Tau mediated neurodegeneration:an insight into Alzheimer′s disease pathology [J]. Neurochemical research,2011,36(8):1329-1335.
[17]? Villemagne VL,Doré V,Bourgeat P,et al. Aβ-amyloid and Tau Imaging in Dementia [J]. Semin Nucl Med,2017, 47(1):75-88.
[18]? Kurbatskaya K,Phillips EC,Croft CL,et al. Upregulation of calpain activity precedes tau phosphorylation and loss of synaptic proteins in Alzheimer′s disease brain [J]. Acta Neuropathol Commun,2016,4:34-48.
[19]? Zuo YC,Li HL,Xiong NX,et al. Overexpression of tau rescues Nogo-66-induced neurite outgrowth inhibition in vitro [J]. Neurosci Bull,2016,32(6):577-584.
[20]? Petrasek T,Prokopova I,Sladek M,et al. Nogo-A-deficient transgenic rats show deficits in higher cognitive functions,decreased anxiety,and altered circadian activity patterns [J]. Front Behav Neurosci,2014,8(90):1-15.
[21]? Mehta NR,Lopez PH,Vyas AA,et al. Gangliosides and Nogo receptors independently mediate myelin-associated glycoprotein inhibition of neurite outgrowth in different nerve cells [J]. J Biol Chem,2007,282(38):27875-27886.
[22]? Zuo YC,Xiong NX,Shen JY,et al. MARK2 rescues Nogo-66-induced inhibition of neurite outgrowth via regulating microtubule-associated proteins in neurons in vitro [J]. Neurochem Res,2016,41(11):2958-2968.
(收稿日期:2019-04-04? 本文編輯:封? ?華)

