以DNA為靶點的釕ギ配合物的合成、晶體結構和抗腫瘤活性
蓋爽爽 藍峻峰 張 鵬, 蔣才云*,, 覃逸明*,,
(1廣西科技師范學院,特色瑤藥資源研究與開發(fā)校級重點實驗室,來賓 545004)
(2廣西科技師范學院食品與生化工程學院,來賓 545004)
Cancer seriously endangers people′s life and safety. In 2018, the World Health Organization reported that cancer was the second leading cause of death,killing about 9.6 million people worldwide[1]. Chemotherapy is a major method widely applied in current clinical cancer treatment[2-3]. Cisplatin is one of the most effective chemotherapeutic drugs approved by the Food and Drug Administration[4]. However, cisplatin is limited in clinical use due to its severe side effects[5-7]. Therefore,a range of metal complexes have been designed and synthesized for the treatment of cancer[8-11], of which ruthenium complexes are the most promising to be the next generation of anticancer agents[12-13].
Many Ru complexes, such as KP-1019 and NAMI-A, have been widely studied due to their excellent clinical efficacy[14-15]. Ru complexes have been applied in various fields of medicinal chemistry against tumors with different biological targets[16-17]. In addition,aroylhydrazones possess excellent biological and pharmaceutical activities, including anticancer, antiinflammatory, and anti-tubercular activities[18]. More importantly, previous studies have been showed that anticancer activity of metal-base complexes derived from aroylhydrazones were higher than that of the ligands alone[19-20]. Hence, the design of aroylhydrazone complexes chelated with ruthenium ion for anticancer treatment has an important prospect.
The primary target of metal-based agents to kill tumors is DNA, which causes amount of DNA lesions,thus preventing transcription and replication[21-22]. Considering the above factors, we designed and synthesized a Ru ギcomplex derived from aroylhydrazones(Scheme 1). Subsequently, the structure of Ruギcomplex was measured via X-ray crystallography and solved. Finally, the anticancer activity of Ru ギcomplex and its effect on DNA were investigatedin vitro.
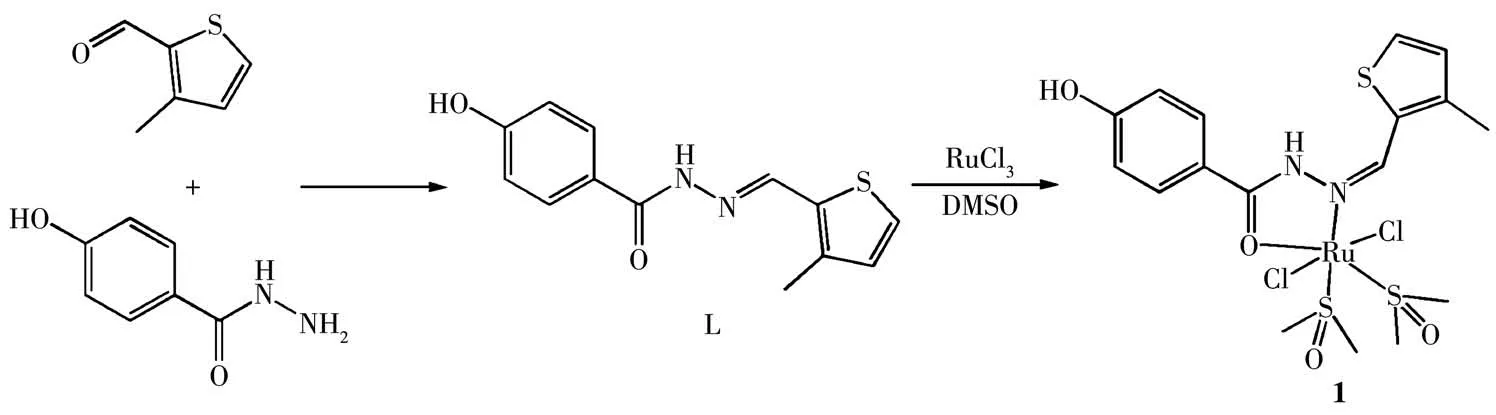
Scheme 1 Synthetic route for[Ru(L)(DMSO)2Cl2](1)
1 Experimental
1.1 Materials and general methods
The chemical reagents and solvent used were analytically pure and purchased from Sigma or Innochem Company. Tumor cell lines were supplied by the Shanghai institute for biochemistry and cell biology.
1.2 Synthesis of[Ru(L)(DMSO)2Cl2](1)
A mixture of 0.1 mmol 4-hydroxybenzhydrazide and 0.1 mmol 3-methyl-2-thiophenecarboxaldehyde were dissolved in 20 mL methanol and refluxed. Then the ligand L was obtained. Subsequently, 0.1 mmol RuCl3and 50 μL DMSO(dimethyl sulfoxide)were added and stirred for another 2 h(Scheme 1).The resulting solution was evaporated at room temperature, and the yellow-brown crystals crystallized after about 7 d. The purity of complex 1 (>95%) was detected by HPLC(Fig.S1). Anal. Calcd. for C17H26Cl2N2O5RuS3(%): C,33.66; H, 4.32; N, 4.62. Found(%): C, 33.72; H, 4.35;N,4.58.
1.3 X-ray crystallography
A single crystal (0.18 mm×0.19 mm×0.22 mm) of 1 was picked out and then measured by a Bruker SMART Apex ⅡCCD diffractometer with graphitemonochromated MoKαradiation (λ=0.071 073 nm).The diffraction data were collected. The structure was solved by direct methods and refined with the XL refinement package using least squares minimization by olex 2.0 software[23]. All non-hydrogen atoms were anisotropically refined. All hydrogen atoms were fixed in calculated positions and refined isotropically. The data is listed in Table 1,and selected bond lengths and angles are listed in Table 2.
CCDC:2080249.
1.4 MTT assays
The human balder cell lines T24 were cultured in 96-well plate and incubated with the selected concentrations of complex 1 for 48 h. Then the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)solution was added to each well and incubated for 4 h.The medium was replaced with 100 μL DMSO. The absorbance was measured at an excitation wavelength of 570 or 630 nm with an enzyme-labelling instrument.
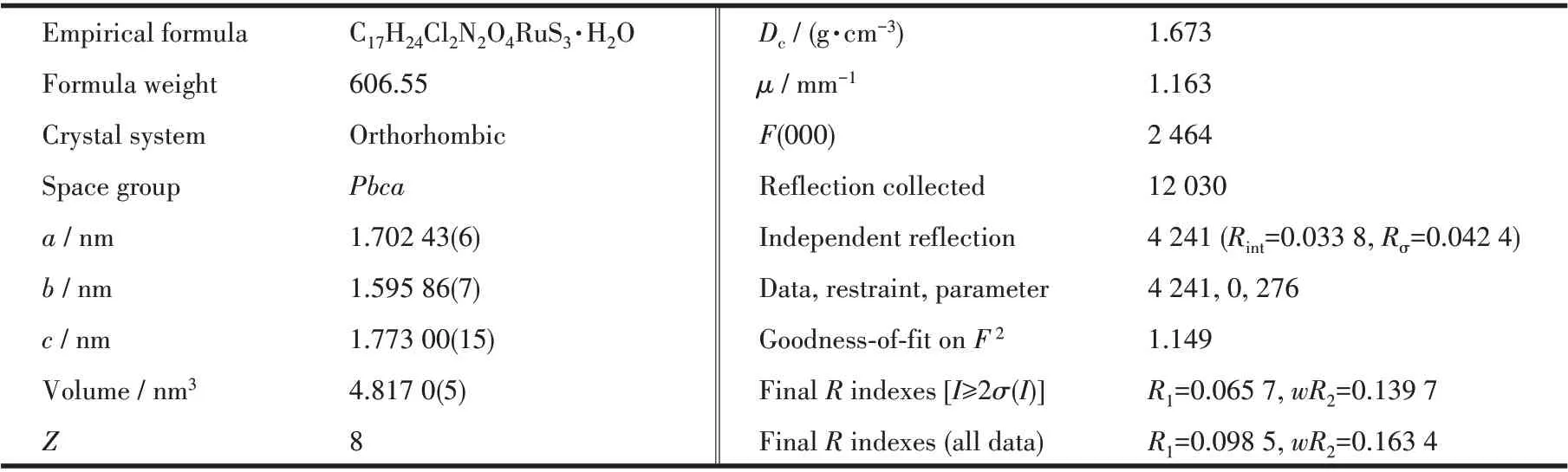
Table 1 Crystal data and structure refinement for complex 1
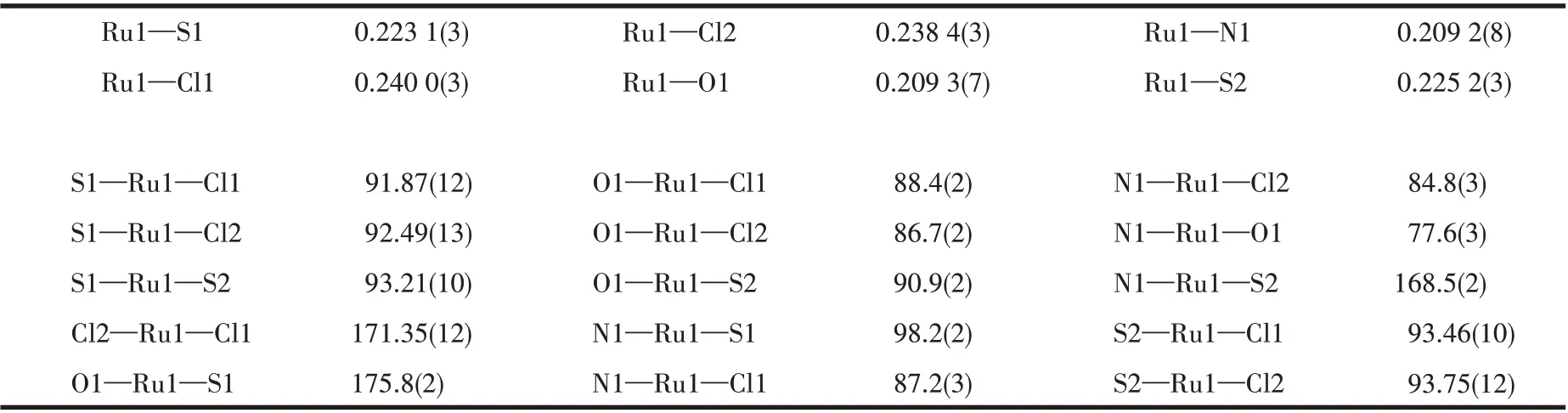
Table 2 Selected bond lengths(nm)and angles(°)of complex 1
1.5 DNA cleavage studies
The super-coiled plasmid pBR322 DNA was incubated with complex 1 for 4 h. Subsequently, agarose gel electrophoresis was employed to isolate pBR322 DNA in the reaction solution. These results were captured by camera for analyzing the ability of complex 1 to cleave DNA.
1.6 Western-blotting
T24 cells were co-incubated with Ruギcomplex for 24 h,and then the cells were harvested and lysed to obtain the proteins. The concentrations of proteins were measured using BCA assay.The proteins were isolated by SDS-polyacrylamide gel, transferred to a PVDF (polyvinylidene fluoride) membrane and blocked with fat-free milk. Then, the membrane was incubated with primary antibodies and secondary antibodies respectively.
1.7 DNA metalation
Two millions T24 cells were placed into 10 cm dishes, and incubated with complex 1 for 24 h. T24 cells were collected by centrifugation and washed once time. Subsequently, DNA was extracted using a PureLink Genomic DNA Mini Kit (Invitrogen). DNA purity was detected by absorbance measurements at 260 and 280 nm.The DNA solution was added 1 mL of concentrated HNO3and 0.5 mL of 30% H2O2. Finally,these samples were detected by ICP-MS.
1.8 Cellular uptake
T24 cells were seeded into 10 cm dishes, and then incubated with complex 1. After 24 h, the cells were collected and washed. Subsequently, the cellular components were isolated and extracted according to the instructions of the mitochondrial isolation kit and the nuclear isolation kit, respectively. The samples were detected by ICP-MS.
1.9 Docking
The binding mode of complex 1 in DNA (PDB ID:1D64) was investigated using AutoDock 4.0. Target receptor(DNA)and complex 1 were prepared via docking protocol and saved into‘PDBQT’format.And then the‘a(chǎn)utogrid’and‘a(chǎn)utodock’were performed,respectively. Energy-scoring function was used to ensure the best Ruギcomplex-DNA pose.
1.10 Intracellular reactive oxygen species (ROS)analysis
T24 cells were cultured into 6-well plates, and treated with complex 1 for 24 h. After incubation, T24 cells were collected and stained in the dark with 2′,7′-dichlorodihydro-fluorescein diacetate (H2DCF-DA) for 30 min. Intracellular ROS generation was detected by flow cytometry.
1.11 Comet assay
T24 cells were incubated with complex 1 for 24 h,and the cells were harvested. 1×104cells were embedded in 80 μL low-gelling-temperature agarose and rapidly pipetted onto a pre-coated microscope slide.These slides were electrophoresed for 20 min, and then stained with PI for 30 min in dark. Finally, the slides were graphed using a laser confocal microscope.
2 Results and discussion
2.1 Structure of 1
X-ray diffraction analysis reveals that 1 crystallizes in the orthorhombic space groupPbca. As shown in Fig.1, the structure of complex 1 is composed of a central Ru ギion, two DMSO molecules, two chloride ions, and a Schiff base ligand L. The central Ru ギforms a six-coordinated environment, which is composed of one N atom (Ru1—N1 0.209 2 nm)and one O atom (Ru1—O1 0.209 3 nm) of HL, two S atoms(Ru1—S1 0.223 1 nm,Ru1—S2 0.225 2 nm)of DMSO and two Cl atoms (Ru1—Cl1 0.240 0 nm, Ru1—Cl2 0.238 4 nm).
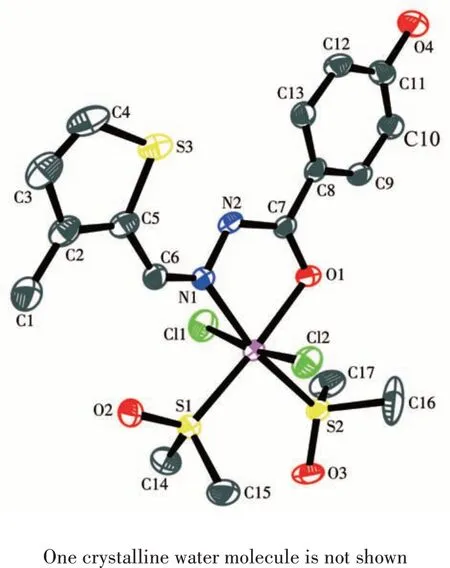
Fig.1 ORTEP diagram of complex 1 showing 30%probability level ellipsoids
2.2 Anticancer activity of 1
The cytotoxicity of 1 against T24 cells was assessed using the MTT assay. The IC50values of HL,RuCl3and DMSO were greater than 50 μmol·L-1.These results indicate that the compounds do not exhibit significant antitumor activity. While the synthesized complex 1 from the ligand and RuCl3exhibited remarkably activity(IC50=15.28 μmol·L-1),as shown in Fig.2.
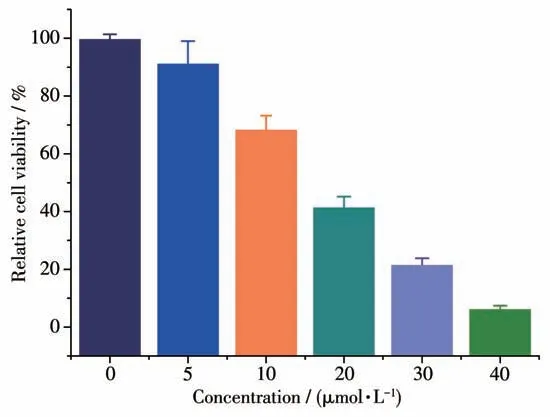
Fig.2 Cytotoxicity of 1 at different concentrations
2.3 Cellular accumulation
The cellular uptake of 1 by T24 cells was measured using ICP-MS.As shown in Fig.3,the contents of ruthenium in cells, nuclei and mitochondria after treatment of 1 were 1.23, 0.28 and 0.11 nmol, respectively.These results suggest that 1 can efficiently enter the cell and accumulate in the nucleus.
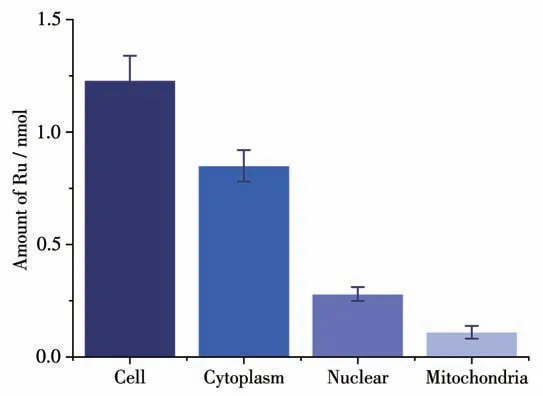
Fig.3 ICP-MS analysis of total Ru contents in cytoplasm,mitochondria,and in nucleus of T24 cells(1×106 cells)treated with 1 for 24 h
2.4 Anti-tumour mechanism of 1
2.4.1 DNA cleavage
DNA is the primary intracellular target of many PT/Ru drugs in the treatment of tumors[21-22]. Hence, the DNA-cleaving activity of 1 was investigated by an agarose gel electrophoresis assay using pBR322 DNA.The control group cannot cleave the PBR322 DNA, whereas 1 can efficiently relax the supercoiled form (FormⅠ) of pBR322 DNA into open circular form (Form Ⅱ)in a dose-dependent manner. These results strongly suggest that complex 1 has a strong effect on DNA(Fig.4).
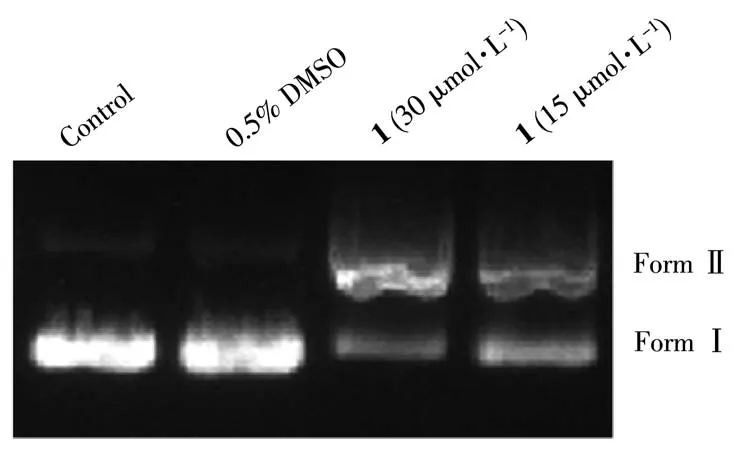
Fig.4 Agarose gel electrophoresis patterns for cleavage of pBR322 DNA by 1
2.4.2 Docking studies
To better understand the mode between B-DNA and 1, molecular docking was carried out for studying the interactions. Complex 1 fitted well into the curved profile of the B-DNA in the minor groove located in the A-T rich region (Fig.5).The energy minimization structure indicates that 1 is stabilized by hydrophobic contact, van der Waals forces and B - DNA functional groups.
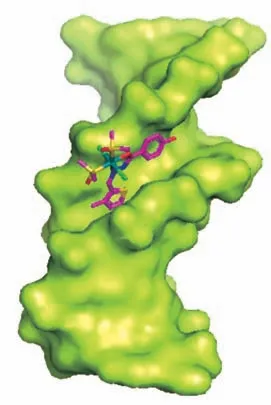
Fig.5 Molecular docked model of 1 with B-DNA
2.4.3 DNA damage studies
To further confirm the ability of 1 acting on DNA in tumor cells, the genomic DNA was extracted from T24 cells after treated with 1. The Ru content in the genomic DNA was at highly level, and the significantly elevated level of Ru content was in a dose-dependent fashion(Fig.6).
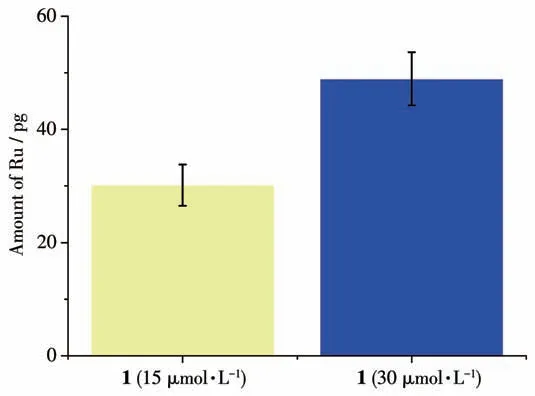
Fig.6 Amount of Ru in genomic DNA(1 μg)of T24 cells after treatment of 1
T24 cells were induced apparent DNA damage co-incubated with complex 1, which was characterized by the tail DNA. The number and length of tail DNA were in positive correlation with the degree of DNA damage. The 1-treated group produced more and longer tail DNA in a dose-dependent manner than the control group(Fig.7).
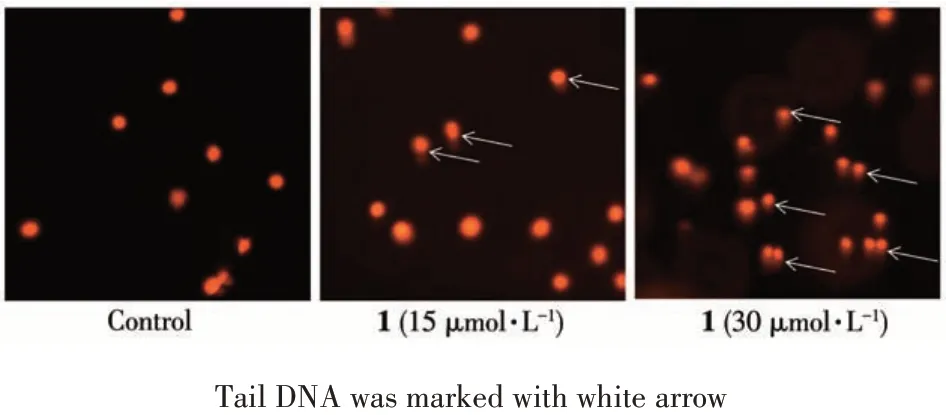
Fig.7 Comet assay for analyzing DNA damage of T24 cells after treatment of 1
Western blotting assays showed that the expression level ofγH2AX (a well-known marker for DNA double-strand breaks) in the control group was kept at lower levels, while that in the treatment group significantly increased (Fig.8). Taken together, these results strongly indicate that direct interaction with DNA is a possible mechanism for complex 1 to play a role in killing tumor cells.
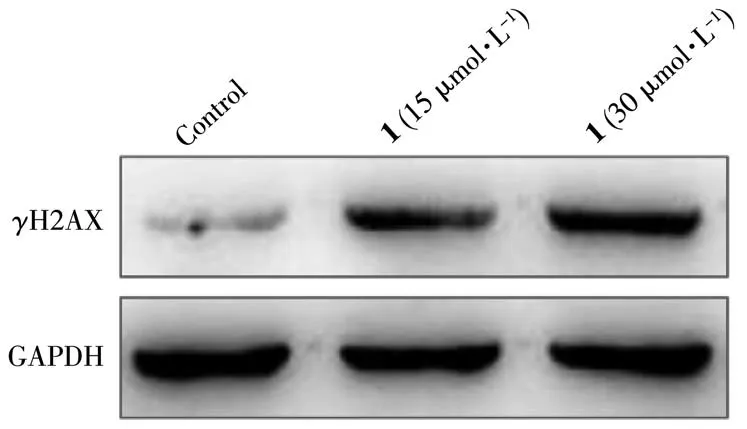
Fig.8 Expression level of γH2AX treated with 1
2.4.4 ROS generation
Generally, ROS is, at least in part, responsible for the cleavage of DNA[24-26]. Therefore, the amount of intracellular ROS after treatment of 1 was investigated by flow cytometry using the DCFH-DA.T24 cells treated with 1 led to a shift in the fluorescence peak to the right compared to the un-treatment group, which indicate that 1 significantly increases intracellular ROS levels in a dose-dependent fashion(Fig.9).
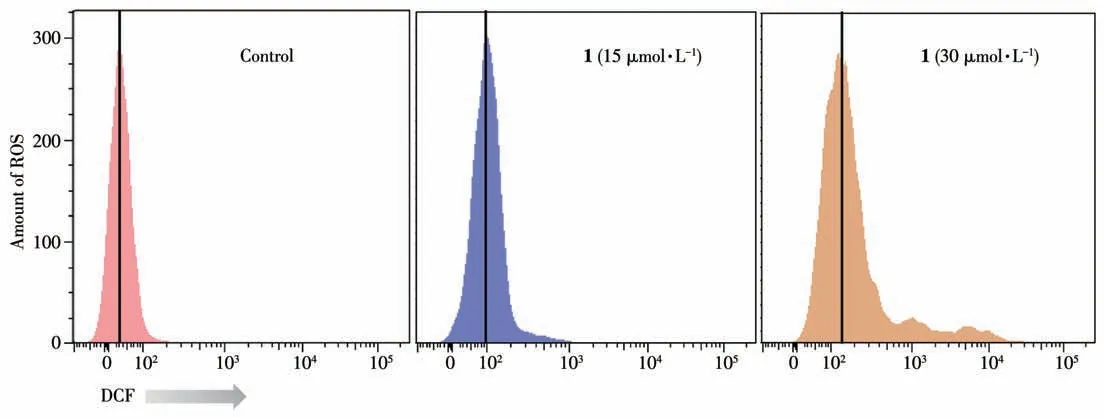
Fig.9 Analysis of ROS levels in T24 cells treated with 1 for 24 h
3 Conclusions
A Ruギcomplex,[Ru(L)(DMSO)2Cl2](1),was synthesized and its anticancer activity was investigated.Complex 1 exhibited effective activities against T24 cells,whereas the ligands had no significant cytotoxicity.Complex 1 is possible to kill tumor cells through the following mechanisms: 1 can bind to DNA bases, form DNA adducts, induce genomic DNA damage, and may block transcription and replication. Additionally, 1 can produce amount of ROS, causing oxidative loss of T24 cells, which leads to tumor cell death. In summary,complex 1 has potential as an anti-cancer chemotherapeutic agent. Our studies may be useful in guiding the development for designing new metal-base antitumor agents.
Supporting information is available at http://www.wjhxxb.cn

