基于雙吡啶酰胺配體的一維汞(Ⅱ)配位聚合物的合成、晶體結構、熒光及蒸氣吸附性質
黃 超 王開騰 陳冬梅 朱必學 盧季紅
(貴州大學,貴州省大環化學及超分子化學重點實驗室,貴陽 550025)
0 Introduction
Coordination polymers(CPs)are generally constructed from organic ligands and inorganic metal ions(or metal clusters)linked by coordination bonds.They have been receiving considerable attention in the past decades not only for their fascinating structures but also for their different applications in the fields of catalysis and photocatalysis,gas adsorption and separation,chemical sensing and detection,etc[1-5].The assembly of CPs with desired structures and functions is primarily depending on many factors,including the kind of organic ligands,the coordination orientation of metal ions,supramolecular weak contacts(π…π stacking and hydrogen bonding interactions),and some other external factors such as the solvent,the material ratio,and the counteranion[6-10].Among the above factors,the type of organic ligands has a remarkable effect on the structures[11-14].Recently,dipyridylamide ligands,with multiple coordination configurations and flexible backbones,have attracted a lot of interest in the construction of various architectures[15-17].CPs based on dipyridylamide ligands reveal that the hydrogen bonds of amide are relatively strong and well understood.Besides,the pyridyl moiety is most popular in the construction of metal-organic networks due to its strong coordination ability.The combination of the two moieties can effectively add extra novelty and dimensionality to the final structures[18-22].
In this work,our studies focus on the dipyridyl building blocks(3-pyridyl or 4-pyridyl rings)for the construction of CPs with diverse structures.Two dipyridylamide ligands were synthesized from the reaction of 9,9'-bis(4-aminophenyl)fluorene with nicotinoyl chloride hydrochloride or isonicotinoyl chloride hydrochloride(Scheme 1),respectively.Then two 1D CPs,{[Hg2(L1)(μ2-I)2I2]·2DMF·H2O}n(1)and{[Hg(L2)I2]·H2O}n(2),were obtained by the assembly of the two ligands with mercury(Ⅱ)iodide.The different configurations of the CPs disclose that the change of coordination sites has an important influence on the formation of different structures.
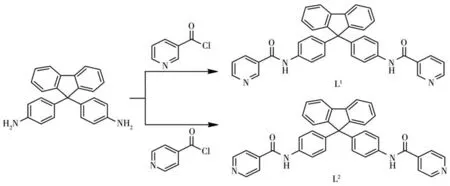
Scheme 1 Synthesis of the two dipyridylamide ligands
1 Experimental
1.1 Materials and physical measurements
All reagents and solvents were commercially available and used as received.1H NMR spectra were recorded in DMSO-d6using a JEOL JNM-ECZ400s spectrometer.Elemental analyses of C,H,and N were performed with a Vario EL Ⅲ elemental analyzer.FT-IR spectra were recorded with KBr discs with a Bruker VERTEX 70 instrument in a range of 4 000-400 cm-1.Powder X-ray diffraction(PXRD)patterns of the complexes were recorded on a Bruker D8 Advance X-ray diffractometer(Cu Kα,λ=0.154 06 nm)operated at 40 kV and 15 mA with a Kβ foil filter,and the data were collected in a 2θ range of 5°-50°with a step size of 0.02°.Thermogravimetric analysis(TGA)was carried out on a PerkinElmer TGA 4000 thermal analyzer in the temperature region of 25-800℃.Solid-state fluorescence spectra were recorded on a Hitachi F-4500 spectrometer.Adsorption isotherms were measured by a Micromeritics ASAP 2020 analyzer.
1.2 Synthesis of the two ligands
Nicotinoyl chloride hydrochloride(0.712 g,4.0 mmol)was dissolved in CH2Cl2(60 mL).Then 9,9'-bis(4-aminophenyl)fluorene(0.697 g,2.0 mmol)and triethylamine(0.5 mL)in CH2Cl2(60 mL)was added dropwise to the above solution.The solution was kept stirring with reflux for 8 h.After filtration,the precipitate was washed with CH2Cl2and treated with a 5% NaHCO3solution.It was then washed in distilled water,dried,and recrystallized from methanol to give yellowish solids of L1.Yield:0.860 g(77%).1H NMR(DMSO-d6,400 MHz):δ 7.08(d,4H,J=8.8 Hz,Ar-H),7.30(t,2H,J=7.2 Hz,Ar-H),7.38(t,2H,J=7.2 Hz,Ar-H),7.44(d,2H,J=7.6 Hz,Ar-H),7.51(dd,2H,J=3.2,4.8 Hz,Py-H),7.61(d,4H,J=8.8 Hz,Ar-H),7.90(d,2H,J=8.0 Hz,Ar-H),8.21(d,2H,J=8.0 Hz,Py-H),8.70(d,2H,J=5.2 Hz,Py-H),9.03(s,2H,Py-H),10.42(s,2H,CONH).Anal.Calcd.for C37H26N4O2(%):C 79.55,H 4.69,N 10.03;Found(%):C 79.59,H 4.74,N 9.98.FT-IR(KBr pellet,cm-1):3 273(w),3 046(w),1 656(s),1 600(s),1 525(s),1 407(w),1 328(s),825(w),740(w),717(w).
The ligand L2was synthesized like L1.Yield:0.838 g(75%).1H NMR(DMSO-d6,400 MHz):δ 7.10(d,4H,J=8.8 Hz,Ar-H),7.30(t,2H,J=7.6 Hz,Ar-H),7.38(t,2H,J=7.2 Hz,Ar-H),7.44(d,2H,J=7.6 Hz,Ar-H),7.61(d,4H,J=8.8 Hz,Ar-H),7.78(d,4H,J=6.4 Hz,Py-H),7.91(d,2H,J=7.6 Hz,Ar-H),8.72(d,4H,J=6.0 Hz,Py-H),10.49(s,2H,CONH).Anal.Calcd.for C37H26N4O2(%):C 79.55,H 4.69,N 10.03;Found(%):C 79.60,H 4.63,N 10.08.FT-IR(KBr pellet,cm-1):3 458(w),3 041(w),1 664(s),1 600(w),1 525(s),1 402(w),1 321(w),830(w),752(w),687(w).
1.3 Synthesis of the two complexes
HgI2(91 mg,0.2 mmol)in methanol(80 mL)was added dropwise with stirring to L1(56 mg,0.1 mmol)in DMF(40 mL)and the solution was refluxed for 20 min.Then the resulting solution was filtered off and allowed to stand at room temperature.Yellow block crystals suitable for X-ray diffraction were obtained after 3 d.The crystals of complex 1 were collected by filtration and air-dried.Yield:106 mg(65.3%).Anal.Calcd.for C43H42Hg2I4N6O5(%):C 31.65,H 2.59,N 5.15;Found(%):C 31.71,H 2.66,N 5.09.FT-IR(KBr pellet,cm-1):3 262(w),3 050(w),1 654(s),1 598(s),1 519(s),1 402(w),1 324(w),827(w),741(w),699(w).
HgI2(91 mg,0.2 mmol)in methanol(80 mL)was added dropwise with stirring to L2(112 mg,0.2 mmol)in DMF(60 mL)and the solution was refluxed for 20 min.Then the same process as that of complex 1 was used to obtain crystals of complex 2.Yield:121 mg(58.7%).Anal.Calcd.for C37H28HgI2N4O3(%):C 43.10,H 2.74,N 5.43;Found(%):C 43.25,H 2.61,N 5.58.FT-IR(KBr pellet,cm-1):3 267(w),3 043(w),1 660(s),1 599(s),1 522(s),1 409(w),1 324(w),829(w),746(w),688(w).
1.4 Crystallographic data collection and structure determination
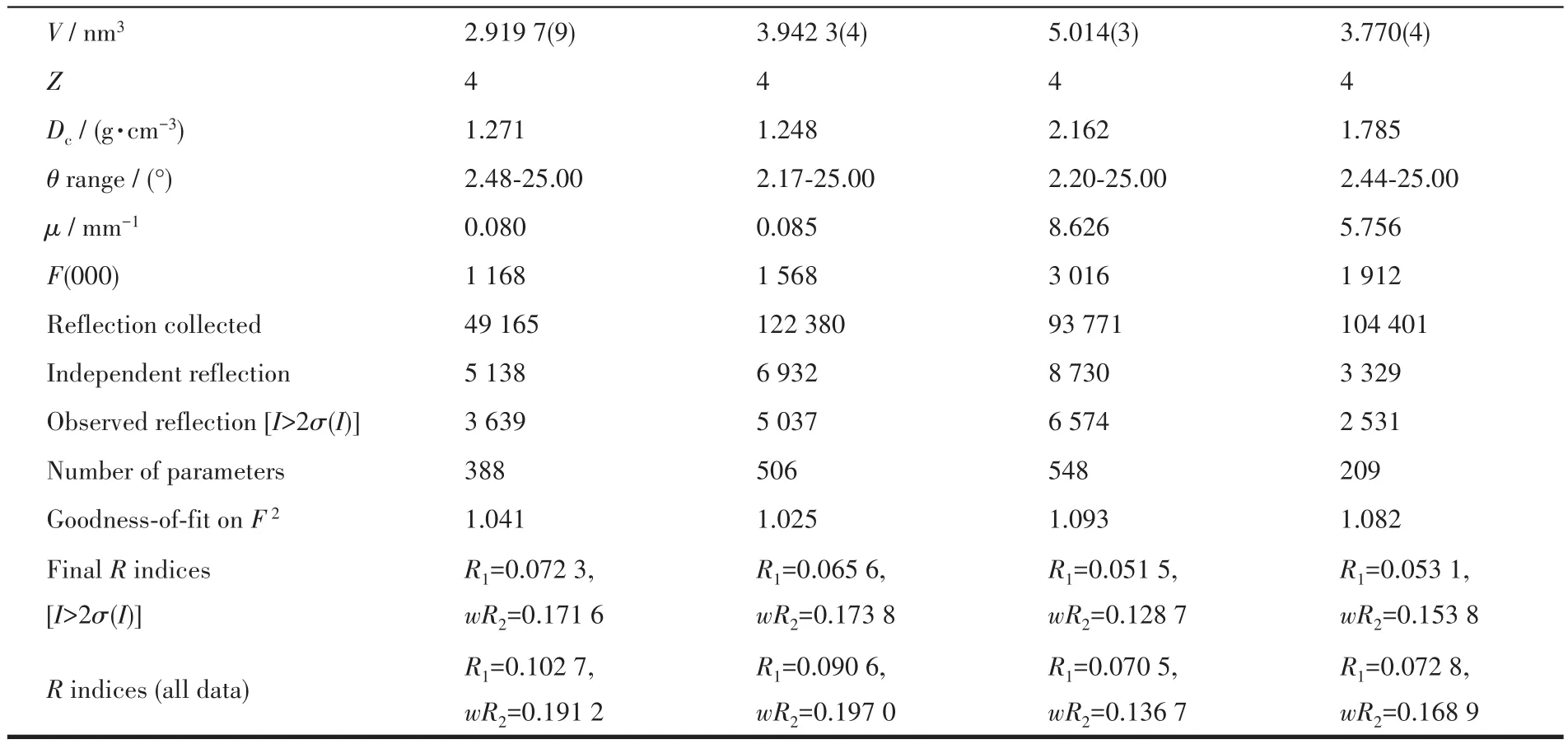
Single crystal X-ray analysis was performed using a Bruker APEX Ⅲ CCD diffractometer equipped with a fine focus sealed tube of monochromatized Mo Kα radiation(λ=0.071 073 nm)at 293(2)K.Data were collected and reduced by smart and saint software in the Bruker package[23].Crystal structures were solved by direct methods and refined by full-matrix leastsquares methods on F2using the OLEX2 program[24].Anisotropic thermal factors were assigned to all the non-hydrogen atoms.The disordered lattice water molecule in complex 2 was removed from the structural data by PLATON/SQUEEZE.Crystallographic data parameters for structural analysis are summarized in Table 1.Selected bond lengths and angles for the complexes are listed in Table S1(Supporting information).The details of the hydrogen bonds for the ligands and complexes are listed in Table S2.

Table 1 Crystallographic data and structure refinement for the ligands and complexes
CCDC:2085335,L1;2085336,L2;2085337,1;2085338,2.
Continued Table 1
2 Results and discussion
2.1 Structural description
2.1.1 Crystal structure of L1and L2
Both the two ligands crystallized from MeOH/DMF via slow evaporation.They both crystallize in the monoclinic crystal system with space group P21/c.The two terminal pyridine rings in L1are parallel to each other(the dihedral angle is about 2°).The dihedral angles between the central fluorene plane and two terminal pyridine rings are 81.18°and 80.32°,respectively(Fig.1a).In the packing structure,each L1molecule is found to be associated with three neighboring molecules via four N—H…O hydrogen bonds between the amide nitrogen atoms and the carbonyl oxygen atoms(Fig.1b).Through these hydrogen bonding interactions,the ligands are linked to form a 2D sheet structure parallel to the ac plane.By contrast,the dihedral angle between the two terminal pyridine rings in L2is about 75°,and the dihedral angles between the fluorene plane and the two pyridine rings are 89.24°and 40.97°,respectively(Fig.2a).In the packing structure,a series of intermolecular hydrogen bonds,such as N—H…O,O—H…O,and N—H…N,are formed among the ligands and the solvent molecules,which results in a 2D sheet structure parallel to the ab plane(Fig.2b).
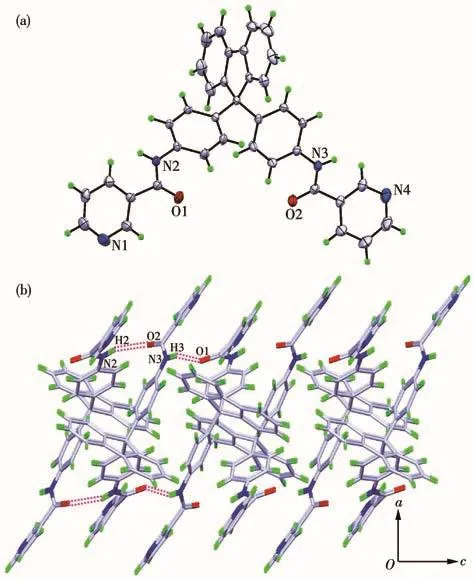
Fig.1 (a)Crystal structure of L1(Ellipsoid probability:30%);(b)2D sheet structure of L1formed viaN—H…O hydrogen bonds
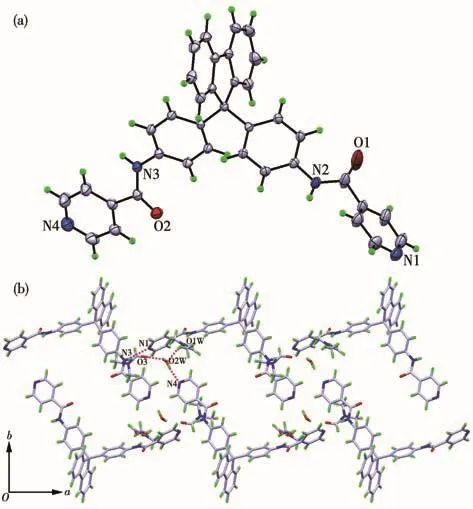
Fig.2 (a)Crystal structure of L2(Ellipsoid probability:30%);(b)2D sheet structure of L2formed via a series of intermolecular hydrogen bonds
2.1.2 Crystal structure of the complexes
Slow evaporation of a solution of ligand L1and HgI2in MeOH/DMF(2∶1,V/V)afforded yellow block crystals of complex 1.Complex 1 belongs to the monoclinic crystal system with space group P21/c.In the complex,each Hg(Ⅱ)is four-coordinated with a distorted tetrahedral geometry deduced by its structural parameter τ4=0.70 for Hg1 center and τ4=0.72 for Hg2 center[25].Each Hg(Ⅱ)ion is coordinated by one 3-pyridyl nitrogen atom and three iodide anions(Fig.3a).Among the three iodide anions,one acts as a terminal ligand and two others act as μ2-bridging ligands between two Hg ions to build up a Hg—(I)2—Hg quadrilateral.The Hg1—N1a and Hg2—N4 bond lengths are 0.242 0(8)and 0.248 1(8)nm,and the Hg-I bond lengths are between 0.258 65(11)and 0.266 22(10)nm,which are consistent with those observed in similar complexes[26-28].The bond angles around the two Hg(Ⅱ)centers vary from 98.17(18)°to 156.77(3)°.The Hg…Hg distances bridged by L1and bridged by the iodide atoms are 1.61 and 0.40 nm(Fig.3b),respectively.The structure of complex 1 can be described as a 1D zigzag chain coordination polymer with a 2∶1 molar ratio of mercury iodide and L1,and diverse intermolecular hydrogen bonds(N—H…O and N—H…N)are formed in the 1D chain.The resulting 1D structures are further linked by C—H…π interactions to give a 2D supramolecular structure parallel to the ac plane(Fig.3c).
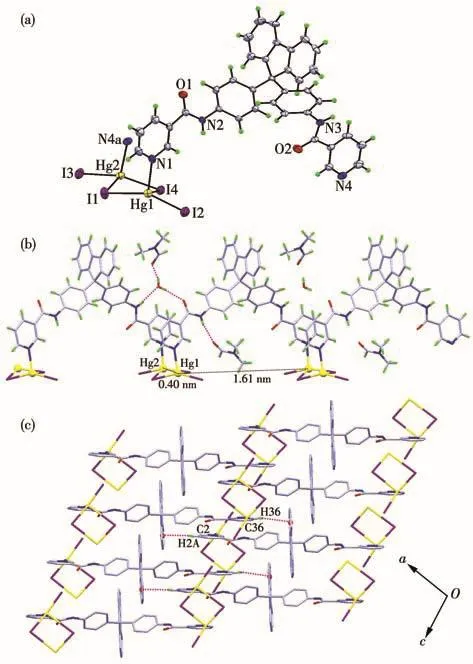
Fig.3 (a)Coordination environment of Hg ions in complex 1(Ellipsoid probability:30%);(b)1D zig-zag chain structure and intermolecular hydrogen bonds in 1;(c)2D supramolecular network of 1 parallel to the ac plane
Complex 2 belongs to the orthorhombic crystal system with the Pnna space group.The complex also exhibits a 1D zig-zag chain structure and each Hg(Ⅱ)is four-coordinated with a distorted tetrahedral geometry deduced by its structural parameter τ4=0.69(Fig.4a).In contrast to complex 1,each Hg(Ⅱ)center in complex 2 is coordinated by two 4-pyridyl nitrogen atoms and two terminal iodide anions,thus the molar ratio of mercury iodide and ligand L2in the 1D chain is 1∶1 instead of 2∶1.The N—Hg bond length is 0.253 5(8)nm and the I—Hg bond length is 0.265 26(13)nm.The bond angles around the Hg(Ⅱ)center are in a range of 95.8(2)°-161.37(5)°,which are consistent with other tetrahedral HgI2complexes[29].The Hg…Hg distances bridged by ligand L2is 2.37 nm(Fig.4b).In the extended structure of complex 2,a 2D supramolecular network is formed parallel to the ab plane via N—H…O hydrogen bonds(Fig.4c).

Fig.4 (a)Coordination environment of Hg ions in complex 2(Ellipsoid probability:30%);(b)1D zig-zag chain structure in complex 2;(c)2D supramolecular network formed via N—H…O hydrogen bonds parallel to the ab plane
2.2 PXRD patterns and thermal stability properties
The experimental PXRD patterns are consistent with the corresponding simulated ones from the single crystal data,which reveals the phase purity of the two complexes(Fig.S4).The difference in reflection intensity may be due to the preferred orientation of the crystalline powder samples.The thermal stability of the two complexes was measured by TGA in the region of 25-800℃(Fig.5).Complex 1 was stable below 105℃,and showed a continuous weight loss in a range of 105-265℃,which is attributed to the loss of the crystalline water and DMF molecules(Obsd.8.2%,Calcd.10.0%).Then the complex began to decompose with a sharp weight loss.Complex 2 was stable below 130℃,and showed a slight weight loss in a range of 130-255℃due to the loss of the water molecules(Obsd.1.7%,Calcd.1.7%).Upon further heating,a sharp weight loss occurred,which is due to the decomposition of the framework.The TGA curves showed that complex 2 was more stable than complex 1,resulting from the lattice solvent molecules.
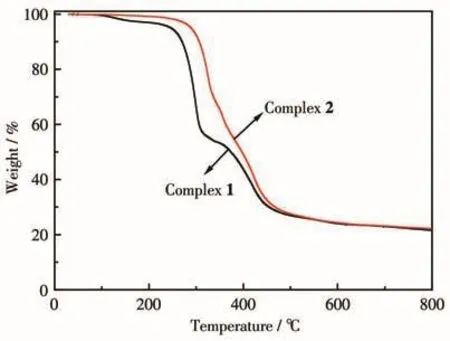
Fig.5 TGA curves of the two complexes
2.3 Luminescence properties
Due to the excellent luminescence properties of d10transition metal complexes,the solid-state luminescence properties of the two complexes together with the two ligands were investigated at room temperature(Fig.6).The main emission peaks were at 518 nm(λex=309 nm)for L1and 522 nm(λex=309 nm)for L2,which can be ascribed to π-π*or n-π*transitions.Complex 1 exhibited maximum luminescence emission at 484 nm(λex=311 nm)and complex 2 exhibited emission peak at 482 nm(λex=310 nm).The blue shifts may be due to the influence of the coordination of mercury ions.Furthermore,the emission intensities of the two complexes exhibited much higher emissions in comparison with the ligands,which may result from that ligand chelation to the metal center effectively enhances the conformational rigidity of the ligand and decreases the loss of energy by radiationless[30].
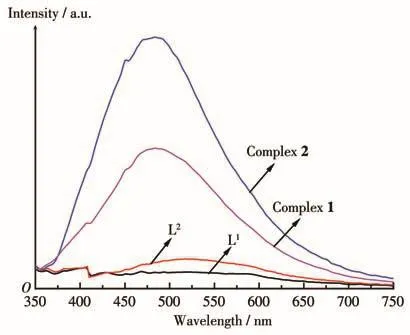
Fig.6 Solid state luminescence emission spectra of the ligands and complexes at room temperature
2.4 Adsorption measurements of the complexes
Considering the hydrogen bonding networks in the 1D chains of the complexes,we attempted to explore the methanol vapor adsorption properties of the complexes at room temperature(298 K).According to the TGA curves of the two complexes,the powder samples of the complexes were activated by drying under a dynamic vacuum at 373 K overnight to get active.The Brunauer-Emmett-Teller(BET)surface areas for complexes 1 and 2 were 148 and 104 m2·g-1,and the Langmuir surface areas were 246 and 181 m2·g-1,respectively.As shown in Fig.7,the vapor adsorption isotherms showed a typical type Ⅲ nature,and the largest quantity adsorbed for complexes 1 and 2 were 4.3 and 3.3 mmol·g-1,which were similar to the complexes in our previous reports[31-33].The structural features of the two complexes may account for the difference in adsorption quantities.
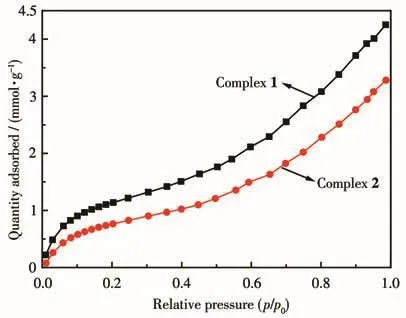
Fig.7 Methanol vapor adsorption isotherms for the two complexes at room temperature
3 Conclusions
In summary,two dipyridylamide ligands derived from 9,9'-bis(4-aminophenyl)fluorene have been designed and synthesized.Furthermore,two 1D zig-zag chain coordination polymers were synthesized from the two ligands with mercury(Ⅱ)iodide,and their crystal structures were determined by single-crystal X-ray diffraction.The mercury centers in the two complexes are four-coordinated but they exhibit different coordination environments.The molar ratio of the ligand and mercury iodide is 1∶2 in complex 1 while 1∶1 in complex 2,which indicates that the nature of the ligands plays an important role in the coordination networks.Besides,the luminescence properties and methanol vapor adsorption measurements for the complexes show that they can be potential materials in related fields.
Supporting information is available at http://www.wjhxxb.cn

