2017—2021年南陽(yáng)市某醫(yī)院胸、腹水培養(yǎng)的病原菌分布及耐藥性分析
王燦燦 王瑩 桑原鋒 矯楊
摘要:目的 分析胸、腹水標(biāo)本中的病原菌分布特點(diǎn)及對(duì)常用抗菌藥物的耐藥性,為臨床醫(yī)師抗感染治療提供參考依據(jù)。方法 回顧性分析2017年1月1日—2021年12月31日南陽(yáng)市第一人民醫(yī)院胸、腹水培養(yǎng)的病原菌分布和耐藥性,使用梅里埃Vitek2–Compact自動(dòng)細(xì)菌鑒定藥敏儀、珠海迪爾DL-96細(xì)菌鑒定分析系統(tǒng)進(jìn)行菌株鑒定和藥敏試驗(yàn),結(jié)果判讀參照當(dāng)年美國(guó)臨床和實(shí)驗(yàn)室標(biāo)準(zhǔn)協(xié)會(huì)(CLSI)發(fā)布的行業(yè)標(biāo)準(zhǔn)。結(jié)果 2 074份胸、腹水培養(yǎng)標(biāo)本,共分離出650株非重復(fù)病原菌,總陽(yáng)性率為31.34%。996份胸水標(biāo)本中分離出病原菌219株,陽(yáng)性檢出率為21.99%;1 078份腹水標(biāo)本中分離出病原菌431株,陽(yáng)性檢出率為39.98%。胸、腹水分離的病原菌中均以革蘭陽(yáng)性菌為主,分別占55.25%、48.96%,均以腸球菌屬和葡萄球菌屬為主;革蘭陰性菌分別占36.07%、43.62%,主要為大腸埃希菌、肺炎克雷伯菌和鮑曼不動(dòng)桿菌;真菌分別占8.68%、7.42%,均以白假絲酵母菌為主。三種主要的革蘭陽(yáng)性球菌對(duì)紅霉素、青霉素的耐藥率高于70.00%,對(duì)萬古霉素、利奈唑胺和替考拉寧的耐藥率為0。大腸埃希菌和肺炎克雷伯菌對(duì)氨芐西林、頭孢唑林的耐藥率最高,超出80.00%,大腸埃希菌對(duì)亞胺培南、美羅培南的耐藥率低于3.00%,肺炎克雷伯菌對(duì)亞胺培南、美羅培南的耐藥率高于25.00%,僅對(duì)替加環(huán)素、多黏菌素B較敏感; 鮑曼不動(dòng)桿菌對(duì)亞胺培南、美羅培南的耐藥率高于40.00%, 但對(duì)頭孢哌酮/舒巴坦、替加環(huán)素比較敏感。結(jié)論 胸、腹腔感染的病原菌復(fù)雜多樣,以革蘭陽(yáng)性菌為主,常見病原菌的耐藥情況相當(dāng)嚴(yán)重。明確胸、腹腔感染的病原菌類別和耐藥情況,對(duì)于臨床醫(yī)師的早期經(jīng)驗(yàn)性抗感染治療和醫(yī)院感染的管控有極其重要的指導(dǎo)意義。
關(guān)鍵詞:胸水;腹水;病原菌;耐藥性;腹腔感染;藥敏試驗(yàn)
中圖分類號(hào):R978.1 ? ? ? ? 文獻(xiàn)標(biāo)志碼:A ? ? ? ? 文章編號(hào):1001-8751(2023)04-0252-04
Distribution and Drug Resistance of Pathogenic Bacteria in Hydrothorax and Ascites of a Hospital in Nanyang City from 2017 to 2021
Wang Can-can, ? Wang Ying, ? Sang Yuan-feng, ? JiaoYang
(Department of Clinical Laboratory, Nanyang First Peoples Hospital, Nanyang 473000)
Abstract:Objective To provide a reference for physicians for anti-infection treatment and to assess the distribution characteristics of pathogens in pleural effusion and ascites samples as well as their resistance to routinely used antibacterial agents. Methods The distribution and drug resistance of pathogenic bacteria cultured in pleural and abdominal water of Nanyang First Peoples Hospital from January 1, 2017, to December 31, 2021 were retrospectively analyzed. The bacterial strains were identified and tested with Meriere Vitek2 - Compact automatic bacterial identification and drug sensitivity analyzer and Zhuhai Deere DL-96 bacterial identification and analysis system. The results were interpreted according to the industry standards issued by American clinical and laboratory standards institute (CLSI). Results A total of 650 strains of non-repeated pathogens were isolated from 2074 specimens of pleural and ascites, with a total positive rate of 31.34%. 219 strains of pathogenic bacteria were isolated from 996 samples of pleural effusion, and the positive detection rate was 21.99%. 431 strains of pathogenic bacteria were isolated from 1078 ascites samples, and the positive detection rate was 39.98%. The main pathogens isolated from hydrothorax and ascites were Gram-positive bacteria, accounting for 55.25% and 48.96%, respectively. The two primary pathogens were Staphylococcus and Enterococcus. Gram-negative bacteria accounted for 36.07% and 43.62%, respectively, mainly Escherichia coli, Klebsiella pneumoniae and Acinetobacter baumannii; fungi accounted for 8.68% and 7.42%, respectively, and Candida albicans predominant. The resistance rates of the three main Gram-positive cocci to erythromycin and penicillin were higher than 70.00%, and the resistance rates to vancomycin, linezolid and teicoplanin were 0.00%. Only tigecycline and polymyxin B were sensitive; Escherichia coli and Klebsiella pneumoniae had the highest drug resistance rates to ampicillin and cefazolin, more than 80.00%; Escherichia coli had the lowest drug resistance rates to imipenem and meropenem, less than 3.00%; and Klebsiella pneumoniae had the highest drug resistance rates to imipenem and meropenem, more than 25.00%. The drug resistance rate of Acinetobacter baumannii to imipenem and meropenem was higher than 40.00%, but it was sensitive to cefoperazone/sulbactam and tigecycline. Conclusion The pathogens of thoracic and abdominal infections are complex and diverse, mainly Gram-positive bacteria, and the drug resistance of common pathogens is quite serious. Defining the pathogen category and drug resistance of thoracoabdominal infection has extremely important guiding significance for clinicians' early empirical anti-infective treatment and management and control of nosocomial infection.
Key words:hydrothorax; ascites; pathogenic bacteria; drug resistance; abdominal infection; susceptibility testing
近幾年隨著微創(chuàng)手術(shù)的大力開展及抗菌藥物的普遍使用,導(dǎo)致胸、腹腔感染的病原菌類別逐漸發(fā)生改變,耐藥情況也日趨嚴(yán)重。胸、腹水標(biāo)本的細(xì)菌培養(yǎng)和藥敏試驗(yàn)對(duì)胸、腹腔感染的診治有著非常重要的臨床意義[1-2]。為了解胸、腹水標(biāo)本中的病原菌類別及對(duì)常用抗菌藥物的耐藥情況,指導(dǎo)臨床科學(xué)合理選用抗菌藥物,本文對(duì)南陽(yáng)市第一人民醫(yī)院2017—2021年胸、腹水標(biāo)本的病原菌分布及其藥敏結(jié)果展開回顧性分析,現(xiàn)報(bào)道如下。
1 資料與方法
1.1 菌株來源
選取2017年1月1日—2021年12月31日南陽(yáng)市第一人民醫(yī)院住院患者的胸、腹水標(biāo)本2 074份,培養(yǎng)分離的650株病原菌,剔除同一患者相同部位分離的重復(fù)菌株。
1.2 方法
標(biāo)本采集與培養(yǎng):按照第四版《全國(guó)臨床檢驗(yàn)操作規(guī)程》[3]中的操作方法進(jìn)行,臨床醫(yī)生嚴(yán)格遵照無菌操作穿刺抽取胸水或腹水,取5~10 mL標(biāo)本注入需氧培養(yǎng)瓶并立即送檢,放置于梅里埃Bact/Alert 120全自動(dòng)血培養(yǎng)儀中。陽(yáng)性報(bào)警時(shí)取瓶?jī)?nèi)液體轉(zhuǎn)種血瓊脂平板,進(jìn)行病原菌鑒定與藥敏試驗(yàn)。病原菌使用梅里埃VITEK2–Compact自動(dòng)細(xì)菌鑒定藥敏儀、珠海迪爾DL-96細(xì)菌鑒定藥敏分析系統(tǒng)進(jìn)行菌株鑒定和藥敏試驗(yàn),參照當(dāng)年美國(guó)臨床和實(shí)驗(yàn)室標(biāo)準(zhǔn)協(xié)會(huì)(CLSI)發(fā)布的行業(yè)標(biāo)準(zhǔn)進(jìn)行結(jié)果判讀。
1.3 質(zhì)控菌株
質(zhì)控菌株為大腸埃希菌ATCC 25922、銅綠假單胞菌ATCC 27853、肺炎克雷伯菌ATCC 49619、金黃色葡萄球菌ATCC 25923、糞腸球菌ATCC 29212,均來源于衛(wèi)生部臨床檢驗(yàn)中心。
1.4 統(tǒng)計(jì)學(xué)處理
應(yīng)用WHONET5.6軟件進(jìn)行統(tǒng)計(jì)分析。
2 結(jié)果
2.1 病原菌檢出情況
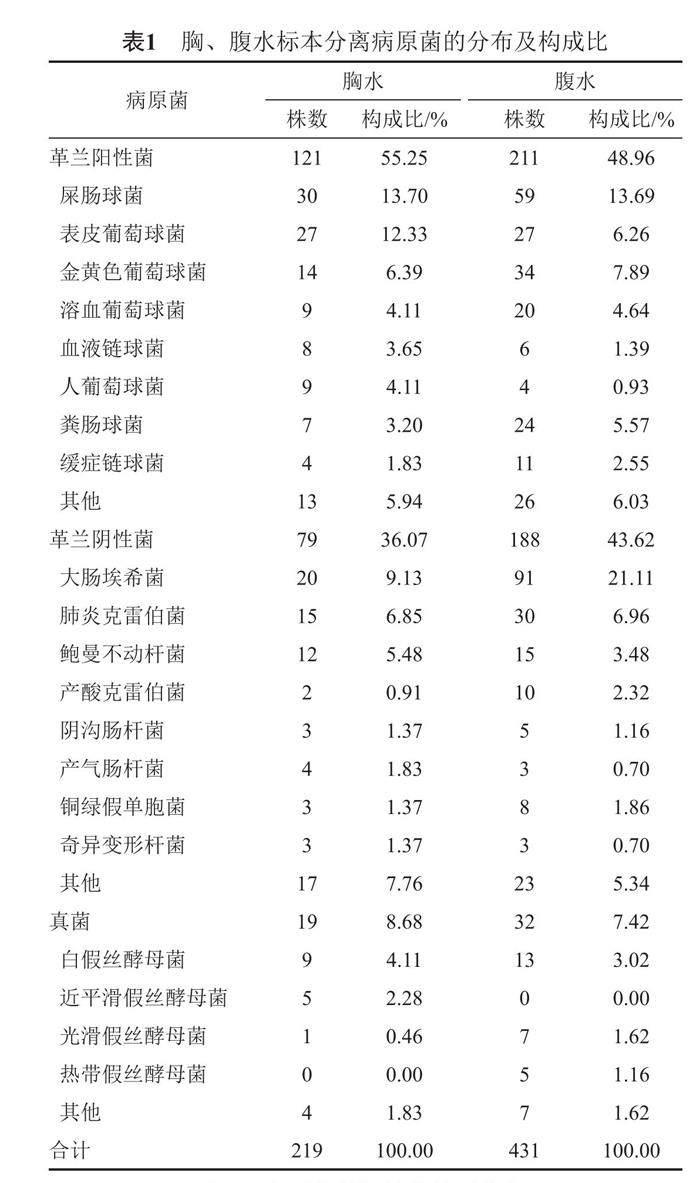
2017—2021年南陽(yáng)市第一人民醫(yī)院收到胸、腹水培養(yǎng)標(biāo)本2 074份,共分離出650株非重復(fù)病原菌,總陽(yáng)性率為31.34%。996份胸水標(biāo)本中分離出病原菌219株,陽(yáng)性檢出率為21.99%,其中革蘭陽(yáng)性球菌121株,構(gòu)成比為55.25%,主要為屎腸球菌、表皮葡萄球菌和金黃色葡萄球菌;革蘭陰性桿菌79株,構(gòu)成比為36.07%,主要為大腸埃希菌、肺炎克雷伯菌和鮑曼不動(dòng)桿菌;真菌19株,構(gòu)成比為8.68%,主要為白假絲酵母菌。1 078份腹水標(biāo)本中分離出病原菌431株,陽(yáng)性檢出率為39.98%,其中革蘭陽(yáng)性球菌211株,構(gòu)成比為48.96%,主要為屎腸球菌、金黃色葡萄球菌和凝固酶陰性葡萄球菌;革蘭陰性桿菌188株,構(gòu)成比為43.62%,主要為大腸埃希菌和肺炎克雷伯菌;真菌32株,構(gòu)成比為7.42%,主要為白假絲酵母菌。見表1。
2.2 主要病原菌對(duì)常用抗菌藥物的耐藥情況
2.2.1 革蘭陽(yáng)性菌中主要病原菌的耐藥性分析
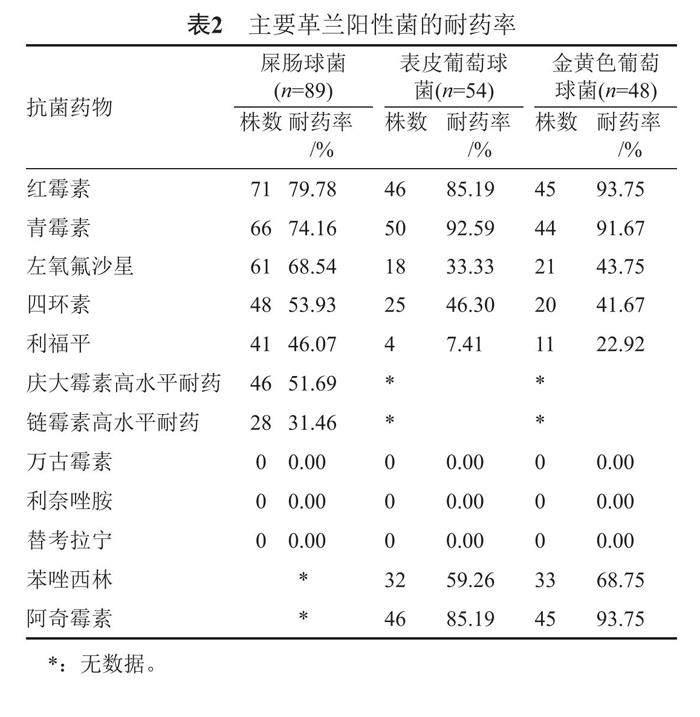
屎腸球菌對(duì)紅霉素、青霉素的耐藥率大于70.00%;表皮葡萄球菌對(duì)青霉素、紅霉素、阿奇霉素的耐藥大于85.00%;金黃色葡萄球菌對(duì)紅霉素、青霉素、阿奇霉素的耐藥率大于90.00%,對(duì)苯唑西林耐藥率為68.75%。未發(fā)現(xiàn)對(duì)萬古霉素、利奈唑胺和替考拉寧耐藥菌株,其耐藥率為0。見表2。
2.2.2 革蘭陰性菌中主要病原菌的耐藥性分析
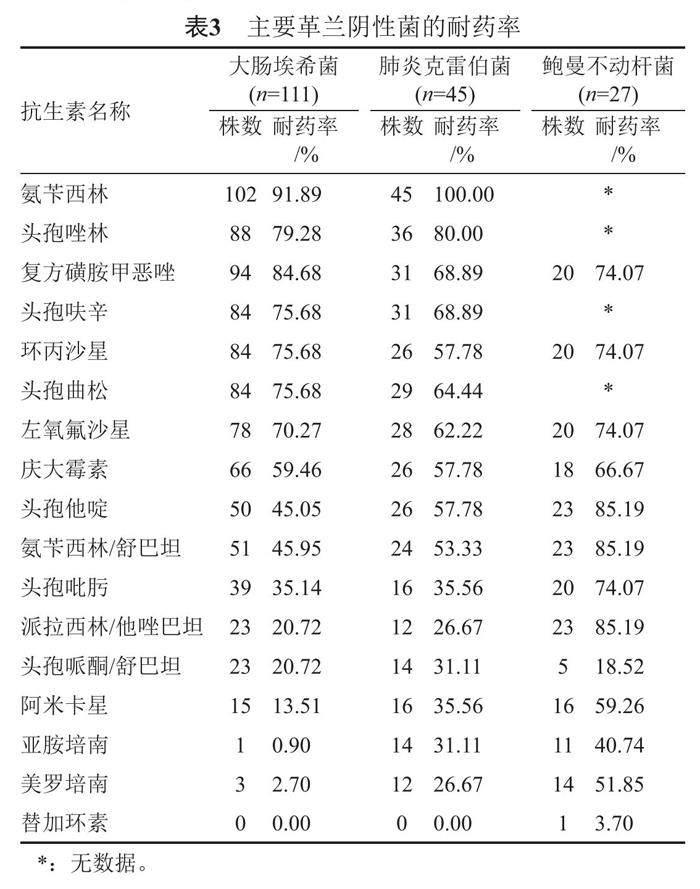
大腸埃希菌對(duì)氨芐西林的耐藥率大于90.00%,對(duì)復(fù)方磺胺甲惡唑、頭孢唑啉、頭孢呋辛、頭孢曲松、環(huán)丙沙星的耐藥率大于70.00%,但對(duì)亞胺培南、美羅培南的耐藥率最低,小于3.00%;肺炎克雷伯菌對(duì)多數(shù)抗菌藥物的耐藥率達(dá)到了50.00%以上,僅對(duì)頭孢哌酮/舒巴坦、哌拉西林/他唑巴坦、亞胺培南、美羅培南的耐藥率低于30.00%;鮑曼不動(dòng)桿菌的耐藥情況最嚴(yán)重,對(duì)亞胺培南、美羅培南的耐藥率已經(jīng)達(dá)到了40.00%以上,但對(duì)頭孢哌酮/舒巴坦、替加環(huán)素耐藥率較低,小于20.00%。見表3。
3 討論
本研究結(jié)果顯示,2017—2021年南陽(yáng)市第一人民醫(yī)院收到的2 074份胸腹水標(biāo)本共分離出650株非重復(fù)病原菌,總陽(yáng)性檢出率為31.34%,高于鄧懋清等[4]的報(bào)道。可能與各種創(chuàng)傷性檢查以及廣譜抗菌藥物的濫用導(dǎo)致細(xì)菌移位引發(fā)感染的機(jī)會(huì)增加,同時(shí)與微生物檢驗(yàn)技術(shù)水平的提高有關(guān)[5]。在胸、腹水分離的病原菌中均以革蘭陽(yáng)性菌為主,分別占55.25%、48.96%,以腸球菌屬和葡萄球菌屬為主;革蘭陰性菌分別占36.07%、43.62%,排名前三的是大腸埃希菌、肺炎克雷伯菌和鮑曼不動(dòng)桿菌;真菌分別占8.68%、7.42%,均以白假絲酵母菌為主,真菌所占比重較往年文獻(xiàn)報(bào)道有升高的趨勢(shì)[6-8],這與臨床上廣譜抗菌藥物長(zhǎng)期反復(fù)使用,特別是第三代頭孢菌素和碳青霉烯類藥物的使用相關(guān),應(yīng)引起臨床注意。以上數(shù)據(jù)不同于張麗娜等及蔡鮮等的報(bào)道[9-10],可見不同地區(qū)、不同醫(yī)院胸腹腔感染的病原菌分布及構(gòu)成比存在差異。
藥敏結(jié)果顯示,屎腸球菌、表皮葡萄球菌和金黃色葡萄球菌對(duì)青霉素類和大環(huán)內(nèi)酯類的耐藥率較高,屎腸球菌對(duì)高濃度慶大霉素和鏈霉素耐藥率分別為51.69%和31.46%,提示聯(lián)合氨基糖苷類治療屎腸球菌感染成功率較低。本研究中MRSA和MRCNS達(dá)到了60.00%以上,應(yīng)引起臨床重視。同時(shí)應(yīng)制定有針對(duì)性的感染防范措施,同時(shí)加強(qiáng)對(duì)病原菌的監(jiān)測(cè),以減少多重耐藥菌株的產(chǎn)生[11]。未發(fā)現(xiàn)對(duì)萬古霉素、利奈唑胺、替考拉寧耐藥株,提示臨床對(duì)于重癥感染患者可選用上述藥物進(jìn)行抗感染治療。革蘭陰性桿菌耐藥情況更加嚴(yán)重,對(duì)各種抗菌藥物呈不同水平的耐藥。本研究中,大腸埃希菌和肺炎克雷伯菌對(duì)青霉素類、第二代及第三代頭孢菌素類和喹諾酮類抗菌藥物的耐藥率較高,在治療時(shí)應(yīng)盡量避免選用上述藥物;大腸埃希菌對(duì)碳青霉烯類耐藥率小于3.00%,臨床上治療多重耐藥大腸埃希菌的感染時(shí)可選用此類藥物,但耐碳青霉烯類肺炎克雷伯菌達(dá)到了25.00%以上,僅對(duì)替加環(huán)素、多黏菌素B較敏感。文獻(xiàn)表明,治療耐碳青霉烯類腸桿菌科細(xì)菌時(shí),聯(lián)合用藥可通過協(xié)同作用或相加作用更快控制感染和遏制耐藥發(fā)生,且聯(lián)合用藥可適當(dāng)降低毒副作用較大藥物的劑量,減少不良反應(yīng),顯著降低病死率[12-14]。鮑曼不動(dòng)桿菌的耐藥情況愈加嚴(yán)峻和復(fù)雜,其耐藥機(jī)制包括產(chǎn)生抗菌藥物滅活酶,外排泵過度表達(dá),作用靶位的改變,外膜蛋白的缺失或改變,形成細(xì)菌生物膜等[15]。本研究中,鮑曼不動(dòng)桿菌的耐藥情況最嚴(yán)重,對(duì)亞胺培南、美羅培南的耐藥率分別為40.74%、51.85%,僅對(duì)頭孢哌酮/舒巴坦、替加環(huán)素比較敏感,臨床醫(yī)師應(yīng)參考藥敏試驗(yàn)結(jié)果,綜合考慮藥物在感染部位的分布濃度等因素,聯(lián)合用藥抗感染治療。
綜上所述,本院胸、腹腔感染的病原菌復(fù)雜多樣,以革蘭陽(yáng)性菌為主,常見病原菌的耐藥性日趨嚴(yán)重。明確胸、腹腔感染的病原菌種類和耐藥情況,對(duì)于臨床醫(yī)師的早期經(jīng)驗(yàn)性抗感染治療和醫(yī)院感染的管控有極其重要的指導(dǎo)意義。臨床醫(yī)師應(yīng)在感染之初,應(yīng)用抗菌藥物之前盡早送檢細(xì)菌培養(yǎng),及時(shí)關(guān)注培養(yǎng)和藥敏結(jié)果,依據(jù)藥敏實(shí)驗(yàn)結(jié)果,及時(shí)調(diào)整治療策略,選擇對(duì)其敏感的抗菌藥物進(jìn)行抗感染治療,可有效提高抗感染治療的成功率。
參 考 文 獻(xiàn)
姚燕珍, 鮑舟君, 陳瓊娜, 等. 胸腔積液中病原菌分布及耐藥情況分析[J]. 中國(guó)衛(wèi)生檢驗(yàn)雜志, 2017, 27(22): 3321-3323.
Skrupky L P, Tellor B R, Mazuski J E. Current strategier for the trement of complicated intra-abdominal infectiongs [J]. Expert Opin Pharmacother, 2013, 14(14): 1933-1947.
尚紅, 王毓三, 申子瑜. 全國(guó)臨床檢驗(yàn)操作規(guī)程[M].4版.北京: 人民衛(wèi)生出版社, 2015.
鄧懋清, 曹春芳, 黃浩南, 等. 胸腹水標(biāo)中的病原菌分布及耐藥性分析[J].實(shí)驗(yàn)與檢驗(yàn)醫(yī)學(xué), 2016, 34(6): 774-776.
臧 婉, 殷 勤, 何建維, 等. 2013-2019年無菌體液病原菌分布及耐藥性分析[J].國(guó)際檢驗(yàn)醫(yī)學(xué)雜志, 2020, 41(1): 65-73.
劉爽, 肖曉光, 林琳. 2013—2015年無菌體液病原菌分布及耐藥性分析[J].檢驗(yàn)醫(yī)學(xué)與臨床, 2017, 14(14): 2038-2041.
李麗, 王佳賀. 2016—2018年中國(guó)醫(yī)科大學(xué)附屬盛京醫(yī)院胸腹水標(biāo)本中病原菌分布及耐藥性分析[J].現(xiàn)代藥物與臨床, 2019, 34(11): 3465-3469.
黃仁剛, 楊興祥, 喻華, 等.腹腔感染病原菌及其耐藥性監(jiān)測(cè)[J]. 中國(guó)感染控制雜志.2015, 14(11): 761-765.
張麗娜, 王術(shù)藝, 么建立, 等.2011至2015年秦皇島市第一醫(yī)院胸腹水病原菌分布及耐藥性監(jiān)測(cè)[J]. 河北醫(yī)藥雜志. 2019, 41(15): 2369-2375.
蔡鮮, 李妍淳, 李金, 等. 腹腔分離標(biāo)本病原體構(gòu)成及耐藥性分析[J]. 中國(guó)抗生素雜志, 2016, 41(10): 782-787.
阮媚超. 無菌體液的細(xì)菌分布和耐藥性監(jiān)測(cè)[J]. 中國(guó)衛(wèi)生檢驗(yàn)雜志, 2017, 27(8): 1187-1189.
Al-Agamy M H, Aljallal A, Radwan H H, et al. Characterization of carbapenemases, ESBLs, and plasmid-mediated quinolone determinants in carbapenem-insensitive Escherichia coli and Klebsiella pneumoniae in Riyadh hospitals [J]. Infect Public Health, 2018, 11(1): 64-68.
陳慧君, 朱齊兵, 葉麗君, 等. 耐碳青霉烯類腸桿菌科細(xì)菌的分布及耐藥性分析[J]. 中國(guó)醫(yī)藥, 2020, 15(6) : 953-956.
Eser F, Yilmaz G R, Guner R, et al. Risk factors for rectal colonization of carbapenem-resistant enterobacteriaceae in a tertiary care hospital: a case-control study from Turkey[J]. Turk J Med Sci, 2019, 49(1):341-346.
藍(lán) 鍇, 季 萍, 王曉明, 等. 2018—2020年多中心耐碳青霉烯類鮑曼不動(dòng)桿菌的耐藥及分布特點(diǎn)[J]. 中國(guó)抗生素雜志, 2021, 46(11): 1026-1030.

