鹽脅迫及復水對棉花葉片生理生化和顯微結構的影響



摘 要:【目的】研究棉花苗期響應鹽脅迫的應答機制,為棉花耐鹽品種篩選提供依據。【方法】對耐鹽型棉花和敏感型棉花三葉期幼苗進行200 mM NaCl脅迫處理,觀察NaCl處理下0、48 h和復水48 h的棉花葉片顯微結構并分析生理生化指標。【結果】耐鹽型和敏感型棉花幼苗在NaCl脅迫0~48 h葉片和莖稈逐漸軟化,敏感型幼苗在NaCl處理下子葉枯萎并凋落,真葉葉緣輕微焦化。NaCl處理下,棉花葉片丙二醛濃度顯著升高,葉綠素含量和超氧化物歧化酶活性均顯著降低。經復水處理,耐鹽型和敏感型棉花均得到一定的恢復,丙二醛濃度降低,而葉綠素含量、超氧化物歧化酶活性和過氧化物酶活性均升高。鹽脅迫條件下,耐鹽型和敏感型的棉花葉片厚度、柵欄組織厚度和海綿組織厚度均下降,且敏感型材料SS型下降幅度較大,與正常條件下差異顯著,但2個材料在復水后均有所恢復,且耐鹽型材料恢復更好。【結論】NaCl處理48 h不僅對棉花的生理生化水平有影響,還誘導細胞和組織發生結構性的改變,復水能有效緩解鹽脅迫下棉花的生理生化水平。耐鹽型棉花材料比敏感型材料具有更強的耐鹽性和復水后恢復更快。
關鍵詞:棉花;耐鹽性;生理生化;顯微結構;復水
中圖分類號:S562 文獻標志碼:A 文章編號:1001-4330(2024)10-2351-07
收稿日期(Received):2024-04-06
基金項目:國家重點研發計劃子項目(2021YFD1900802-4)
作者簡介:趙康(1998-),男,新疆人,碩士研究生,研究方向為作物遺傳育種,(E-mail)zhaokang07@yeah.net
通訊作者:高文偉(1973-),男,新疆人,教授,博士,碩士生/博士生導師,研究方向作物遺傳育種,(E-mail)280594606@qq.com
0 引 言
【研究意義】土壤鹽漬化嚴重影響糧食安全[1-2] 。全球約有80×104 hm2的灌溉土壤受到土壤鹽漬化的影響,約占總灌溉面積的40%[3]。據估計至2050年世界上50%的農業用地將會發生不同程度的土壤鹽漬化[4]。我國干旱和半干旱地區次生鹽漬化發生面積逐年增加[5]。我國新疆降水稀少,蒸發量大,土壤中無機鹽聚集,濃度升高,容易發生土壤鹽漬化[6]。鹽堿土是不同鹽漬化土壤的總稱,包括鹽土、堿土、堿化土壤和其他表現出不同程度鹽堿化的土壤[7-8]。鹽土含有高含量中性鹽,主要包括NaCl和Na2SO4,通過影響滲透平衡、離子毒害、營養吸收合成和呼吸影響作物生長及發育等[9-13]。棉花(Gossypium hirsutum L.)屬錦葵科,是一種具有較好抗旱、耐鹽、耐瘠薄能力的農作物,是開發利用鹽堿地的“先鋒作物”[14]。棉花也是我國主要的經濟作物之一[15-16]。高鹽脅迫影響葉綠素合成,使得幼苗生長緩慢,且葉片畸形生長,子葉難以平展[17]。棉花幼苗二葉期、三葉期時,體內已開始花芽分化,對鹽較敏感[18]。通常鹽脅迫下的棉花植株與干旱脅迫下的植株相似,表現出葉緣焦化、葉片顏色變暗、子葉脫落和植株萎蔫等癥狀,由于土壤溶液中的滲透壓增加,導致生理干旱和離子積累,阻礙養分和水分的吸收[19]。因此,提高棉花對鹽堿環境的耐受性尤為重要。【前人研究進展】關于耐鹽機理研究,前人從膜脂過氧化與抗氧化酶,如丙二醛(MDA)、過氧化氫酶(CAT) 、超氧化物歧化酶(SOD)和過氧化物酶(POD)等進行了研究[20]。鹽脅迫下植物細胞內過量累積Na+,誘導活性氧(ROS)過度產生,并啟動膜脂過氧化和膜脂脫脂作用,導致膜蛋白和膜脂損失,從而破壞膜結構,影響生長發育[21-22]。植物也會將葉肉細胞中過多的Na+轉運至葉脈,減少細胞質中的Na+毒害,因此植物葉綠素合成受阻,進而降低葉綠素含量[23-24]。為了適應鹽脅迫,植物展現出了多種適應性策略[6]。耐鹽型植物在鹽脅迫下可能會增加葉片厚度[25]和根系發育[26],以提高吸收水分和營養元素。牧草Imperata cylindrica(L.Raeuschel)的鹽田生態型較正常無鹽環境生態型,鹽堿脅迫下角質層增厚,葉片肉質化,柵欄組織發達,氣孔下陷[27]。【本研究切入點】植物在鹽脅迫下的顯微結構和生理生化變化可反映該植物對鹽的敏感性,而鹽脅迫對棉花解剖結構的影響研究較少[28]。鹽脅迫下植物復水是恢復其表型、生理和基因表達的有效措施。復水可以降低植物受鹽度影響的程度,但是目前有關復水如何影響植物應對鹽脅迫的研究還比較有限[29],因此,棉花的耐鹽性仍有待探究。【擬解決的關鍵問題】試驗通過形態學、生理及微觀結構觀察,研究棉花對鹽脅迫的響應及其復水后的適應性,為探究棉花耐鹽機理提供理論參考。
新疆農業科學第61卷 第10期趙 康等:鹽脅迫及復水對棉花葉片生理生化和顯微結構的影響
1 材料與方法
1.1 材 料
選用2個耐鹽性差異較大的棉花材料[30]:鹽敏感型52-128(salt sensitive,SS)和耐鹽型涇斯棉(salt tolerant,ST),將種子用5%次氯酸鈉(NaClO)消毒20 min,無菌水沖洗3次,在發芽盒(12.4 cm×17.5 cm)中播種,置于受控條件下(儲存在晝夜溫度26℃/18℃,相對濕度65%,晝夜時長為8 h/16 h,光照強度12 000 lx的人工氣候箱中)。待子葉完整展開后,轉移置于1/2 Hoaglands營養液培養至三葉期。對棉花三葉期幼苗在200 mmol/L NaCl溶液中生長48 h,于在1/2 Hoagland營養液中培養48 h(RW,re-watering)。在0、48 h、復水48 h(0、48和營養液中培養即復水48 h)分別取樣,幼苗為3個生物學重復,每個重復6株,共收集18個樣本。
1.2 方 法
1.2.1 生理生化指標測定
葉綠素采用便攜式葉綠素儀(SPAD-502Plus)分別測定不同處理下棉株最大功能葉。采用硫代巴比妥酸比色法、氮藍四唑光還原法和愈創木酚-過氧化氫法,分別測定丙二醛的含量、超氧化物歧化酶活性和過氧化物酶活性[31]。
1.2.2 葉片顯微結構觀察
采用垂直葉脈切法,將不同處理的葉片分別置于FAA固定液中。固定狀態良好后,進行修剪、脫水、包埋、切片、染色、封片及鏡檢合格樣片,并用番紅-固綠染色液染片。用3DHIST型ECH(Hungary)生產的全景切片掃描儀(PANNORAMICDESK/MIDI/250/1000和CaseViewer2.2)采集和掃描瀏覽圖像,通過Media Cybemetics(美國)生產的Image-Pro Plus 6.0分析。測量每張切片中5處葉片厚度、上表皮厚度、柵欄組織厚度、海棉組織厚度和下表皮厚度。
1.3 數據處理
采用Excel 2020整理分析試驗數據,采用SPSS型 26.0進行差異性檢驗,通過GraphPad Prism繪制分組比較圖。
2 結果與分析
2.1 棉花幼苗鹽脅迫和復水后表型變化
研究表明,ST型和SS型幼苗在NaCl脅迫0~48 h中葉片和莖稈逐漸軟化,子葉在48 h萎蔫嚴重。SS型幼苗在48 h子葉枯萎并凋落,真葉葉緣輕微焦化。復水后,ST型生長得到緩解,SS型真葉枯萎。圖1
2.2 棉花葉片對鹽脅迫和復水后生理生化的響應
研究表明,NaCl 48 h處理下,ST型和SS型幼苗丙二醛濃度顯著升高,葉綠素含量、超氧化物歧化酶活性和過氧化物酶活性均降低,鹽脅迫影響了棉株正常的生長發育。ST型和SS型的丙二醛濃度分別較 0 h (CK)升高36.07%和64.61%,復水48 h后ST型幼苗丙二醛濃度較CK變化不明顯,而SS型幼苗丙二醛濃度較CK升高11.54%。鹽脅迫顯著影響了細胞質膜過氧化水平,而復水對ST型的緩解效果優于SS型。ST型和SS型幼苗的葉綠素含量,鹽脅迫下分別較CK降低10.29%和13.89%,復水后較CK分別降低5.23%和10.36%。鹽脅迫顯著抑制了棉花葉綠素合成,復水也顯著改善了ST型和SS型的葉綠素合成。ST型的超氧化物歧化酶活性和過氧化物酶活性,在鹽脅迫下分別降低3.06%和12.98%,復水后,分別較CK降低0.36%和12.51%。SS型的超氧化物歧化酶活性和過氧化物酶活性,在鹽脅迫下分別顯著降低27.31%和22.46%,復水后較CK顯著降低39.79%和36.67%。鹽脅迫下ST型活性氧清除能力強,SS型活性氧清除能力強,且復水并未改善SS型的活性氧清除能力。圖2
2.3 鹽脅迫和復水后棉花葉片顯微結構
研究表明,正常條件下ST型和SS型幼苗切片染色較深,顯微結構排列較緊密,泡狀細胞形如單層不規則的四邊形,并緊密排列;鹽脅迫下泡狀細胞呈卵圓形,柵欄組織和海綿組織細胞排列較松散,葉片變得軟化。復水后泡狀細胞形如長卵形,葉片較平展,逐漸恢復正常生長。圖3
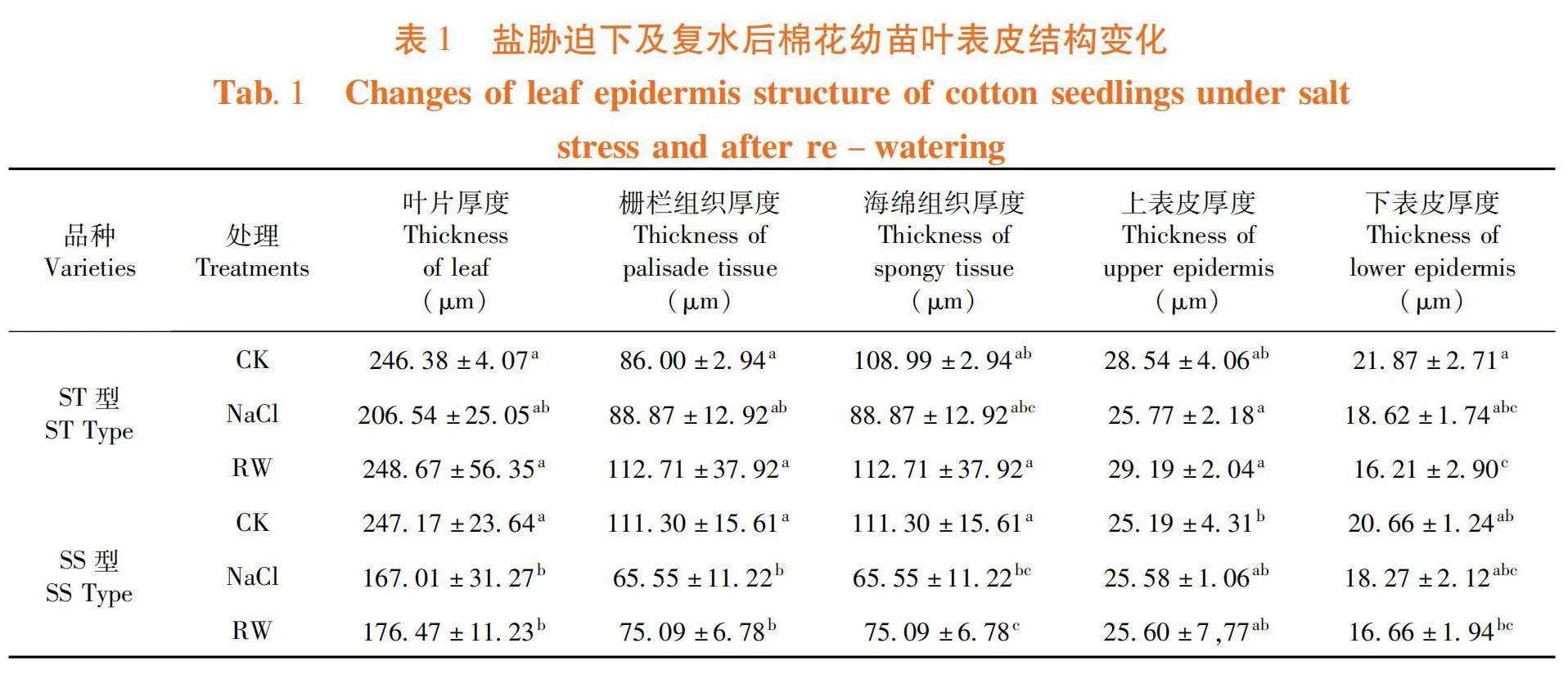
在正常條件下,ST型和SS型棉花幼苗的葉表皮結構差異不顯著;NaCl脅迫條件下,ST型和SS型的葉片厚度、柵欄組織厚度和海綿組織厚度均下降,在復水后有所恢復。ST型棉花幼苗經過NaCl處理后,葉片厚度、柵欄組織厚度和海綿組織厚度較對照分別降低16.17%、13.92%和18.46%;SS型的葉片厚度、柵欄組織厚度和海綿組織厚度較對照分別顯著降低32.43%、30.13%和41.10%。與對照差異顯著。表1
3 討 論
3.1 鹽通過限制植物對水分的吸收而滲透損害,并導致植物細胞中積累過高離子含量脅迫[32]。棉花亦進化出一系列應對鹽度和其他環境壓力的反應,包括形態、生理、生化和分子過程來應對環境脅迫,可避免脅迫后恢復,使植物能夠在非生物脅迫下生存[33]。耐鹽材料與鹽敏感材料的區別可能就在于耐鹽材料在遇到外部高鹽脅迫的時候,相對鹽敏感材料有一個快速適應、生理間和耐受力[34]。NaCl 48h處理下,棉花幼苗葉片受損傷嚴重,ST型和SS型幼苗葉片的丙二醛濃度顯著升高,葉綠素含量和超氧化物歧化酶活性均顯著降低。經過復水處理后,ST型和SS型得到一定的恢復,丙二醛濃度降低,葉綠素含量、超氧化物歧化酶活性和過氧化物酶活性均升高。與袁雨豪[34]的研究結果相類似,耐鹽型材料比敏感型材料表現出更大的耐鹽性和復水后更快的恢復。
3.2 鹽分不僅改變植物的代謝機制, 而且影響植物的正常生長, 尤其是植物的形態學和解剖學[35],葉片是高等植物光合作用的中心,在遭受逆境脅迫時,植物葉片的表型、生理、解剖結構等性狀均能及時響應[36]。棉花Na+區隔化研究表明,陸地棉葉片腺毛泌鹽是棉花耐鹽的具體表現之一[37]。葉片的顯微結構特征是植物適應鹽堿脅迫的一個重要方面,在NaCl脅迫下, 棉花葉片各組成細胞失水收縮,葉片橫切面厚度變薄,柵欄組織等細胞形態受到影響[28]。研究中正常條件下棉花葉片顯微結構排列較緊密,泡狀細胞形如單層不規則的四邊形,并緊密排列;鹽脅迫下泡狀細胞呈卵圓形,柵欄組織和海綿組織細胞排列較松散,葉片變得軟化。復水后泡狀細胞形如長卵形,葉片較平展,逐漸恢復正常生長。在正常條件下,ST型和SS型棉花幼苗的葉表皮結構差異不顯著;NaCl脅迫條件下,ST型和SS型的葉片厚度、柵欄組織厚度和海綿組織厚度均下降,在復水后ST型得到了更好的恢復。
4 結 論
NaCl處理下,棉花葉片丙二醛濃度顯著升高,葉綠素含量和超氧化物歧化酶活性均顯著降低。經復水處理,耐鹽型和敏感型均得到一定的恢復,丙二醛濃度降低,葉綠素含量、超氧化物歧化酶活性和過氧化物酶活性均升高。鹽脅迫不僅對棉花的生理生化水平有影響,還誘導細胞和組織發生結構性的改變,復水可有效緩解鹽脅迫下棉花的生理生化水平。耐鹽型材料比敏感型材料表現出更大的耐鹽性和復水后更快的恢復。
參考文獻(References)
[1]Li Q, Song J. Analysis of widely targeted metabolites of the euhalophyte Suaeda salsa under saline conditions provides new insights into salt tolerance and nutritional value in halophytic species[J].BMC Plant Biology, 2019, 19(1): 388.
[2] Wang J, Jiang X, Zhao C F, et al. Transcriptomic and metabolomic analysis reveals the role of CoA in the salt tolerance of Zygophyllum spp[J].BMC Plant Biology, 2020, 20(1): 9.
[3] Li X W, Zheng H L, Wu W S, et al. QTL mapping and candidate gene analysis for alkali tolerance in japonica rice at the bud stage based on linkage mapping and genome-wide association study[J].Rice, 2020, 13(1): 48.
[4] 趙起越, 夏夜, 鄒本東. 土壤鹽漬化成因危害及恢復[J].農業與技術, 2022, 42(11): 115-119.
ZHAO Qiyue, XIA Ye, ZOU Bendong. Causes, harm and recovery of soil salinization[J].Agriculture and Technology, 2022, 42(11): 115-119.
[5] 努爾沙吾列·哈斯木漢. 新疆土壤鹽漬化成因及其防治對策[J].科學技術創新, 2020, (9): 52-53.
Nuershawulie Hasimuhan. Causes of soil salinization in Xinjiang and its control countermeasures[J].Scientific and Technological Innovation, 2020, (9): 52-53.
[6] Zhao H X, Gu B J, Chen D C, et al. Physicochemical properties and salinization characteristics of soils in coastal land reclamation areas: a case study of China-Singapore Tianjin Eco-City[J].Heliyon, 2022, 8(12): e12629.
[7]Rogers P P, Llamas M R, Martínez-Cortina L. Water Crisis: Myth Or Reality[M].Taylor and Francis, CRC Press.
[8] van Zelm E, Zhang Y X, Testerink C. Salt tolerance mechanisms of plants[J].Annual Review of Plant Biology, 2020, 71: 403-433.
[9] Zhang B L, Chen X G, Lu X K, et al. Transcriptome analysis of Gossypium hirsutum L. reveals different mechanisms among NaCl, NaOH and Na2CO3 stress tolerance[J].Scientific Reports, 2018, 8: 13527.
[10] Chen W C, Cui P J, Sun H Y, et al. Comparative effects of salt and alkali stresses on organic acid accumulation and ionic balance of seabuckthorn (Hippophae rhamnoides L.)[J].Industrial Crops and Products, 2009, 30(3): 351-358.
[11] Byrt C S, Munns R, Burton R A, et al. Root cell wall solutions for crop plants in saline soils[J].Plant Science, 2018, 269: 47-55.
[12] Geng G, Li R R, Stevanato P, et al. Physiological and transcriptome analysis of sugar beet reveals different mechanisms of response to neutral salt and alkaline salt stresses[J].Frontiers in Plant Science, 2020, 11: 571864.
[13] 盧秀茹, 賈肖月, 牛佳慧. 中國棉花產業發展現狀及展望[J].中國農業科學, 2018, 51(1): 26-36.
LU Xiuru, JIA Xiaoyue, NIU Jiahui. The present situation and prospects of cotton industry development in China[J].Scientia Agricultura Sinica, 2018, 51(1): 26-36.
[14] 史曉玲. 國家、生態、技術、市場——棉花與魯西北社會變遷(1906-2006)[D].濟南: 山東大學, 2020.
SHI Xiaoling. Country, Ecology, Technology and Market: Cotton and Social changes in Northwest Shandong(1906-2006)[D].Jinan: Shandong University, 2020.
[15] 吳方衛, 張錦華. 絲綢之路經濟帶農牧業合作的空間、潛力與中國農業“走出去” 策略[J].科學發展, 2016, (4): 76-81.
WU Fangwei, ZHANG Jinhua. Space and potential for husbandry cooperation in silk road economic belt and “going out” strategy of Chinese agriculture[J].Scientific Development, 2016, (4): 76-81.
[16] 蘇瑩, 郭安慧, 華金平. 棉花耐鹽性鑒定方法探討[J].中國農業大學學報, 2021, 26(12): 11-19.
SU Ying, GUO Anhui, HUA Jinping. Strategies for evaluation the salt tolerance in cotton[J].Journal of China Agricultural University, 2021, 26(12): 11-19.
[17] 孫小芳, 劉友良, 陳沁. 棉花耐鹽性研究進展[J].棉花學報, 1998, 10(3): 118-124.
SUN Xiaofang, LIU Youliang, CHEN Qin. Recent progresses in studies on salinity tolerence in cotton[J].Cotton Science, 1998, 10(3): 118-124.
[18]Maryum Zahra, Luqman Tahira, Nadeem Sahar, et al. An overview of salinity stress, mechanism of salinity tolerance and strategies for its management in cottonamp;#13;[J].Frontiers in Plant Science, 2022, 13.
[19]Fu H Q,Yang Y Q. How Plants Tolerate Salt Stress[J], 2023, 45(7): .5914-5934.
[20] 毛桂蓮, 許興, 徐兆楨. 植物耐鹽生理生化研究進展[J].中國生態農業學報, 2004, 12(1): 43-46.
MAO Guilian, XU Xing, XU Zhaozhen. Advances in physiological and biochemical research of salt tolerance in plant[J].Chinese Journal of Eco-Agriculture, 2004, 12(1): 43-46.
[21] Hasanuzzaman M, Oku H, Nahar K, et al. Nitric oxide-induced saltstress tolerance in plants: ROS metabolism, signaling, and molecular interactions[J].Plant Biotechnology Reports, 2018, 12(2): 77-92.
[22] 段慧榮, 周學輝, 胡靜, 等. 高等植物K+吸收及轉運的分子機制研究進展[J].草業學報, 2019, 28(9): 174-191.
DUAN Huirong, ZHOU Xuehui, HU Jing, et al. Advances in understanding molecular mechanisms of K+ uptake and transport in higher plants[J].Acta Prataculturae Sinica, 2019, 28(9): 174-191.
[23] Hauser F, Horie T. A conserved primary salt tolerance mechanism mediated by HKT transporters: a mechanism for sodium exclusion and maintenance of high K(+)/Na(+) ratio in leaves during salinity stress[J].Plant, Cell amp; Environment, 2010, 33(4): 552-565.
[24] 李瑞強, 王玉祥, 孫玉蘭, 等. 鹽脅迫對無芒雀麥幼苗葉片形態及解剖結構的影響[J].草地學報, 2022, 30(6): 1450-1459.
LI Ruiqiang, WANG Yuxiang, SUN Yulan, et al. Effects of salt stress on leaf morphology and anatomical structure of Bromus inermis seedlings[J].Acta Agrestia Sinica, 2022, 30(6): 1450-1459.
[25] 李雙男, 郭慧娟, 侯振安. 不同鹽堿脅迫對棉花離子組穩態及Na+相關基因表達影響[J].棉花學報, 2019, 31(6): 515-528.
LI Shuangnan, GUO Huijuan, HOU Zhen’an. Ionic homeostasis and expression of Na+ related genes of cotton under different salt and alkali stresses[J].Cotton Science, 2019, 31(6): 515-528.
[26] Hameed M, Ashraf M, Naz N. Anatomical adaptations to salinity in cogon grass[Imperata cylindrica (L.) Raeuschel]from the Salt Range, Pakistan[J].Plant and Soil, 2009, 322(1): 229-238.
[27] 趙海燕, 王建設, 劉林強, 等. 海島棉苗期鹽脅迫下形態學和生理學指標變化[J].中國農業科學, 2017, 50(18): 3494-3505.
ZHAO Haiyan, WANG Jianshe, LIU Linqiang, et al. Morphological and physiological mechanism of salt tolerance in Gossypium barbadense to salt stress at seedling stage[J].Scientia Agricultura Sinica, 2017, 50(18): 3494-3505.
[28] Azeem A, Wu Y Y, Xing D K, et al. Photosynthetic response of two okra cultivars under salt stress and re-watering[J].Journal of Plant Interactions, 2017, 12(1): 67-77.
[29] Du L, Cai C P, Wu S, et al. Evaluation and exploration of favorable QTL alleles for salt stress related traits in cotton cultivars (G. hirsutum L.)[J].PLoS One, 2016, 11(3): e0151076.
[30] 范蓉. 基于生理指標與基因表達量評價棉花抗旱耐鹽性[D].烏魯木齊: 新疆農業大學, 2020.
FAN Rong. Evaluation of Cotton Drought and Salt Tolerance Based on Physiological Indexes and Gene Expression[D].Urumqi: Xinjiang Agricultural University, 2020.
[31] 李娜. 不同基因型大白菜對鹽堿脅迫的響應特性[D].泰安: 山東農業大學, 2022.
LI Na. Response Characteristics of Different Genotype Chinese Cabbages to Salt and Alkali Stress[D].Taian: Shandong Agricultural University, 2022.
[32] 葉武威. 棉花種質的耐鹽性及其耐鹽基因表達的研究[D].北京: 中國農業科學院, 2007.
YE Wuwei. Study on the Salinity Resistance and Resistance Gene Expression in Cotton Germplasm[D].Beijing: Chinese Academy of Agricultural Sciences, 2007.
[33] 王德龍. 鹽脅迫下棉花細胞壁重塑相關基因GhEXLB1與GhGRP1功能研究[D].烏魯木齊: 新疆農業大學, 2021.
WANG Delong. Study on the Function of GhEXLB1 and GhGRP1 Genes Related to Cell Wall Remodeling in Cotton under Salt Stress[D].Urumqi: Xinjiang Agricultural University, 2021.
[34] 袁雨豪. 鹽脅迫下糜子的生理響應及適應機制研究[D].楊凌: 西北農林科技大學, 2022.
YUAN Yuhao. Study on Physiological Response and Adaptive Mechanism of Broomcorn Millet(Panicum Miliaceum L.)Under Salt Stress[D].Yangling: Northwest A amp; F University, 2022.
[35] 石亞飛, 閔煒芳, 擺小蓉, 等. 外源物調節堿脅迫水稻生理特性及相關基因表達的效應[J].植物營養與肥料學報, 2023, 29(5): 813-825.
SHI Yafei, MIN Weifang, BAI Xiaorong, et al. Effects of exogenous regulatory substances on physiological characteristics and gene expression of rice seedlings under alkali stress[J].Journal of Plant Nutrition and Fertilizers, 2023, 29(5): 813-825.
[36] Zhao B Q, Liu Q Y, Wang B S, et al. Roles of phytohormones and their signaling pathways in leaf development and stress responses[J].Journal of Agricultural and Food Chemistry, 2021, 69(12): 3566-3584.
[37] 彭振. 棉花苗期耐鹽和耐熱的生理機制及其基因轉錄調控分析[D].雅安: 四川農業大學, 2016.
PENG Zhen. The Gene Transcriptional Regulation Analysis and Physiology Mechanism of Salt Tolerance and Thermotolerance at Seedling Stage in Upland Cotton[D].Yaan: Sichuan Agricultural University, 2016.
Effects of salt stress and re-watering on the physiology,
biochemistry and microstructure of cotton leaf structure
ZHAO Kang1, CHENG Rongrong2,PANG Bo1, ZHANG Mengyuan1, ZHANG Ru1,
WANG Yongpan1, YANG Zhining1,WANG Zhi2,WANG Honggang1, GAO Wenwei1
(1. College of Agronomy, Xinjiang Agricultural University, Urumqi 830052, China; 2. No. 3 Exploration Institute of Geology and Mineral Resources, Yantai Shandong 264000, China;3.Center of Urumqi Comprehensive Survey Natural Resources, Urumqi 830057, China)
Abstract:【Objective】 To study the response mechanism of cotton seedlings in response to salt stress, and to provide experimental basis for the screening of salt-tolerant varieties of cotton. 【Methods】 Salt-tolerant and sensitive cotton seedlings were treated with 200 mM NaCl at the three-leaf stage, and the microstructures of cotton leaves were observed, and physiological and biochemical indexes were analyzed at 0, 48 h and 48 h of re-watering under NaCl treatment.【Results】 Salt-tolerant and sensitive seedlings gradually softened their leaves and stalks under NaCl stress from 0-48 h. Sensitive seedlings withered and faded their cotyledons and slightly scorched the margins of the true leaves under NaCl treatment. The malondialdehyde concentration of cotton leaves was significantly elevated, and the chlorophyll content and the activity of superoxide dismutase were significantly reduced under NaCl treatment. After re-watering, both salt-tolerant and sensitive types were somewhat restored, malondialdehyde concentration was reduced, and chlorophyll content, superoxide dismutase activity and peroxidase activity were increased.Under salt stress conditions, leaf thickness, fenestrated tissue thickness and spongy tissue thickness of salt-tolerant and sensitive types decreased, and the SS type of sensitive material decreased more, and the difference was significant compared with normal conditions, but both materials recovered after re-watering, and the salt-tolerant material recovered better. 【Conclusion】 NaCl treatment for 48 h not only has an effect on the physiological and biochemical levels of cotton, but also induces the cellular activity of superoxide dismutase and oxidase activity. levels, but also induces structural changes in cells and tissues, and re-watering can effectively alleviate the physiological and biochemical levels of cotton under salt stress. Salt-tolerant materials have stronger salt tolerance and faster recovery after re-watering than sensitive materials.
Key words: cotton; salt tolerance; physiology and biochemistry; microstructure; re-watering
Fund projects:National key research and development plan sub-project(2021YFD1900802-4)
Correspondence author: GAO Wenwei (1973-), male, from Xinjiang, Professor, Ph.D., Master/Doctoral' s supervisor instructor, research direction:crop genetics and breeding, (E-mail) 280594606@qq.com

