摻鎂鈦酸鋇納米棒的水熱合成和光催化性能
林 雪關(guān)慶豐李海波巴春華王丹丹孟繁駿
(1江蘇大學(xué)材料科學(xué)與工程學(xué)院,鎮(zhèn)江 212013)
(2吉林師范大學(xué)化學(xué)學(xué)院,環(huán)境友好材料制備與應(yīng)用教育部重點(diǎn)實(shí)驗(yàn)室,四平 136000)
(3吉林師范大學(xué)物理學(xué)院,四平 136000)
摻鎂鈦酸鋇納米棒的水熱合成和光催化性能
林 雪1,2關(guān)慶豐*,1李海波3巴春華2王丹丹2孟繁駿2
(1江蘇大學(xué)材料科學(xué)與工程學(xué)院,鎮(zhèn)江 212013)
(2吉林師范大學(xué)化學(xué)學(xué)院,環(huán)境友好材料制備與應(yīng)用教育部重點(diǎn)實(shí)驗(yàn)室,四平 136000)
(3吉林師范大學(xué)物理學(xué)院,四平 136000)
用水熱法制備摻鎂鈦酸鋇(Ba1-xMgxTiO3(x=0,0.10,0.20,0.30,0.40),BMT)納米粉體。運(yùn)用X射線衍射儀(XRD)、場發(fā)射掃描電子顯微鏡(FESEM)、透射電子顯微鏡(TEM)、紫外可見漫反射光譜技術(shù)(DRS)等手段對樣品進(jìn)行了表征,并在可見光照射下于溶液中考察了其光催化降解甲基橙反應(yīng)活性。結(jié)果表明,通過控制氫氧根濃度可以得到不同形貌的納米粉體。基于不同條件下制備的樣品的微結(jié)構(gòu)分析,提出了這些不同形貌的形成機(jī)制。制備出的BMT材料的帶隙能約為2.61 eV。光催化反應(yīng)結(jié)果表明BMT的光催化活性比摻氮TiO2高得多。OH-濃度為8 mol·L-1時制備的BMT納米棒光催化效率最高,經(jīng)可見光照射360 min,濃度為0.01 mmol·L-1甲基橙溶液的降解率可達(dá)到93.0%,且循環(huán)使用4次后,其光催化活性并沒有明顯降低,表明BMT是一種穩(wěn)定有效的可見光催化劑.
鈦酸鋇;摻鎂;納米結(jié)構(gòu);水熱合成;光催化降解;可見光照射
The worldwide demand for clean and renewable energy sourceshasencouraged a greatdealof research activities and development in the field of solar energy in the last twenty years.Solar energy as a clean energy is inexhaustible in supply and always available for use.Therefore,high efficient catalyst,photochemical cell and solar cell have become the hotspot of scientific research.Since the discovery of the photocatalytic splitting of water on the TiO2electrodes by Fujishima and Honda (1972),the application of semiconductor photocatalysts on degradation of pollutants has received great attention[1-6].Among all photocatalysts,TiO2attractsthemost attention due to its chemical stability,low cost,nontoxicity, and high photocatalytic activity[7-10].However,the band gap energy of the TiO2is 3.2 eV.It absorbs only the ultraviolet light (λ≤386.5 nm)which only accounts for about 4%of the sunlight.In orderto improve the efficiency ofthe sunlight utilization,the development of photocatalysts with high activity under a wide range of visible light is highly desirable[11-14].
Barium titanate(BaTiO3,BTO)is one of the most widely used ferroelectric materials and has been extensively studied[15-16].Nevertheless there have been few studies about the visible light-induced photocatalytic activity ofBTO.In heterogeneous photocatalysis,the morphology of the catalyst plays an important role in catalytic activity[17-24].One-dimensionalnanostructuresphotocatalystshave attracted extensive attentions in environmental remediation due to their great specific surface area[25-27].Furthermore,Metal elements doping is one of the typical approaches to extend the spectral response of semiconductor photocatalysts by providing defect states in the band gap[28].
Methyl orange (MO)has a variety of uses in texitiles,paper,pulp and environment thus causing toxicity problems.Many efforts have been made to study the photodegradation of MO.In this work,we have introduced a low-temperature solution-phase route without the use of any surfactants or templates to synthesize Magnesium doped barium titanate(BMT)photocatalysts with well controlled shapes. As stimulated by the promising applications,the synthesis of BMT nanorods is a subject of considerable research interest.There are two significant aspects of the work described in this paper.Firstly,the synthesis of shape-controlled BMT nanostructures has been found to be extremely evasive to date.Hence,the facile and template-free hydrothermal synthesis of BMT nanostructures with well controlled shapes should be an important progress that may inspire subsequent catalytic materials synthesis.Secondly,the test of BMT nanorods associated opticalproperties and photocatalytic activities in the degradation of MO under visible-light irradiation has been rarely reported.Hence,this work may be of interest to both materials scientists and those working in the area of catalyst design.
1 Experimental
1.1 Preparation of BMT photocatalysts
All the chemicals were analytical grade(purchased from Shanghai Chemical Industrial Company)and used withoutfurther purification.Barium chloride (BaCl2·2H2O),magnesium chloride(MgCl2·6H2O)and titanium tetrachloride (TiCl4)were chosen as starting materials.The BMT synthesis was as follows:a 10 mL TiCl4was dissolved in 50 mL cold H2O under vigorous stirring,then mixed with BaCl2and MgCl2.The concentration of the alkali solution was adjusted using NaOH.Before being transferred to a 20 mL stainless steel autoclave,the solution mixture was prepared under an ultrasonic water bath for 30 min in order to avoid the premature formation of bismuth titanate nuclei induced by NaOH and kept at a filling ratio of 70% (volume ratio).The autoclave was kept at 230℃for 24 h,and cooled to room temperature after the reaction.The precipitates were washed with deionized water and ethanol three times,separately.The final products were dried at 100℃for 2 h in a vacuum box.The sample prepared for comparison is N-TiO2synthesized according to Hou et al[29].
1.2 Characterization of BMT photocatalysts
The crystalstructures ofthe samples werecharacterized by X-ray diffraction (XRD)on a PE D/max 2500 X-ray diffractometer(Cu Kα radiation,λ=0.154 18 nm),employing a scanning rate of 4.00°·min-1,in the 2θ range from 20°to 80°.The operation voltage and current were maintained at 40 kV and 30 mA,respectively.The sizes and morphologies of the resulting samples were studied by field emission scanning electron microscope (FESEM,JEOL JSM-6700F)ataccelerating voltage of10 kV and transmission electron microscope (TEM,JEOL JEM-2100F)ataccelerating voltageof200 kV.The chemical composition of the compound was determined by scanning electron microscope-X-ray energy dispersion spectrum (SEM-EDX).The UV-Vis diffuse reflectance spectra (DRS)were recorded for the dry-pressed disk sample using a scan UV-Vis spectrophotometer (SHIMADZU UV-2550)equipped with an integrating sphere.Raman spectrum of BMT was obtained by a micro laser Raman spectrometer(LabRam inva).Raman spectrum was excited with 514 nm line of an Ar+laser at an incident power of 20 mW.Theopticalpropertywasobtained bythe photoluminescence (PL)measurement using HR800 LabRam Infinity Spectro photometer excited by a continuous He-Cd laser with a wavelength of 325 nm at a power of 50 mW.
1.3 Photocatalytic activities test
The photocatalytic degradation ofMO was employed to evaluate the photocatalytic activities of the samples.A 1 000 W halogen lamp with a 420 nm cutoff filter was employed to provide visible light irradiation.0.10 g of photocatalyst was added to 100 mL MO solution (0.01 mmol·L-1).Before irradiation,the suspensions were magnetically stirred in the dark for30 min to ensure the adsorption-desorption equilibrium between the photocatalysts and MO.Then the solution was exposed to visible light irradiation under magnetic stirring.At given time intervals,4 mL of suspension was sampled and centrifuged to remove the photocatalyst particles.Then,the catalyst-free dye solution was analyzed by a UV-2550 spectrometer to record intensity of the maximum band at 460 nm in the UV-Vis absorption spectra.
2 Results and discussion
2.1 Characterization analysis
2.1.1 XRD analysis
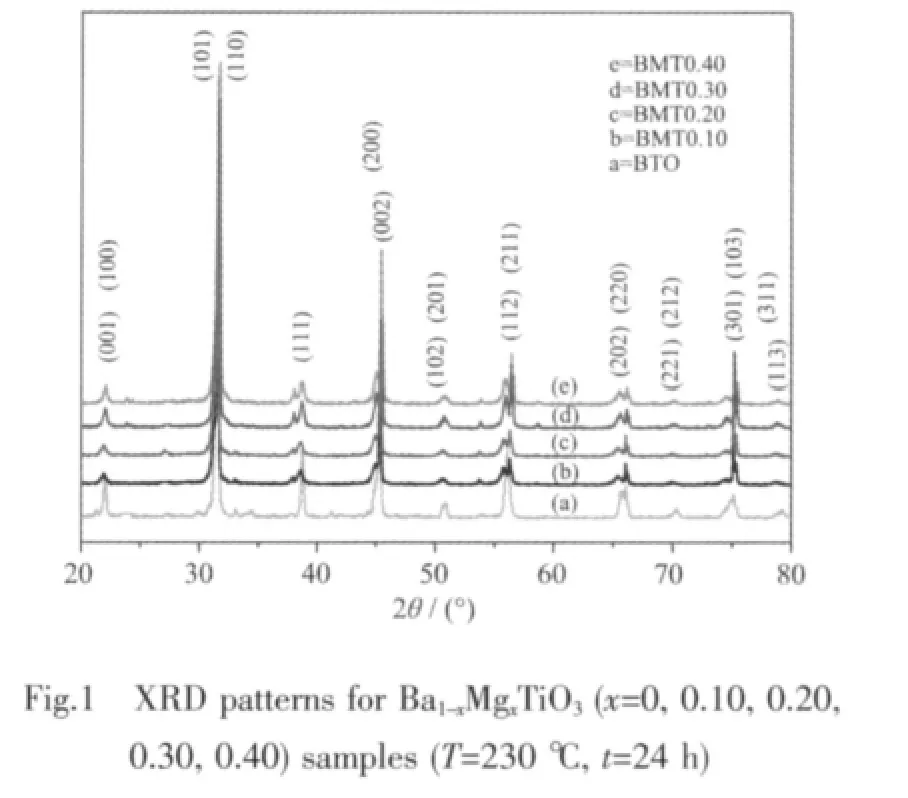
XRD patterns of BaTiO3powders with various Mg doping synthesized by hydrothermal method are shown in Fig.1.It is indicated that the phase composition of all the powders with various Mg doping consists of a single phase with a tetragonal BaTiO3,consistent with the literature[30-31].No peaks of impurities are detected from the patterns.The strong and sharp peaks indicate high crystallinities of BMT samples.
2.1.2 Microstructures analysis
The influence of OH-concentration on the morphology ofBa0.96Mg0.4TiO3(BMT)crystals was present using NaOH with concentration in the range of 3 to 8 mol·L-1.Fig.2(a)~2(c)show FESEM images of the samples prepared at various OH-concentration.It can be seen that BMT products prepared at OH-concentration of 3 mol·L-1are composed of nano-sized particles with average size of 50 nm,and each particle is nearly spherical in shape (as shown in Fig.2(a)).Fig.2 (b)shows BMT sample obtained at OH-concentration of 5 mol·L-1.It also can be observed that there are BMT nanorods with width of 50~100 nm and lengths up to several micrometres.In addition to nanorods,nano-sized particles are also observed in the sample prepared at OH-concentration of 5 mol·L-1.However,when the OH-concentration is further increased to 8 mol·L-1,a large number of BMT nanorods are formed, accompanied by thedisappearance of the spherical particles(as illustrated in
Fig.2 (c)).These resultsindicate thatOH-concentration plays an important role in determining final morphologies of BMT.Namely,morphology of the BMT powders can be controlled through varying the concentration of OH-.The relationship between the OH-concentration and morphology of BMT crystals will be discussed later.

Further structure details of BMT nanorods were studied by TEM,as shown in Fig.3.It can be observed thateach nanorod iswith an average diameter of 50 nm and a side length over 1 μm through their axialdirection.In particular,no branching is observed for each nanorod,which implies that the nanorods are grown from a spontaneous nucleation and with high crystal perfection.
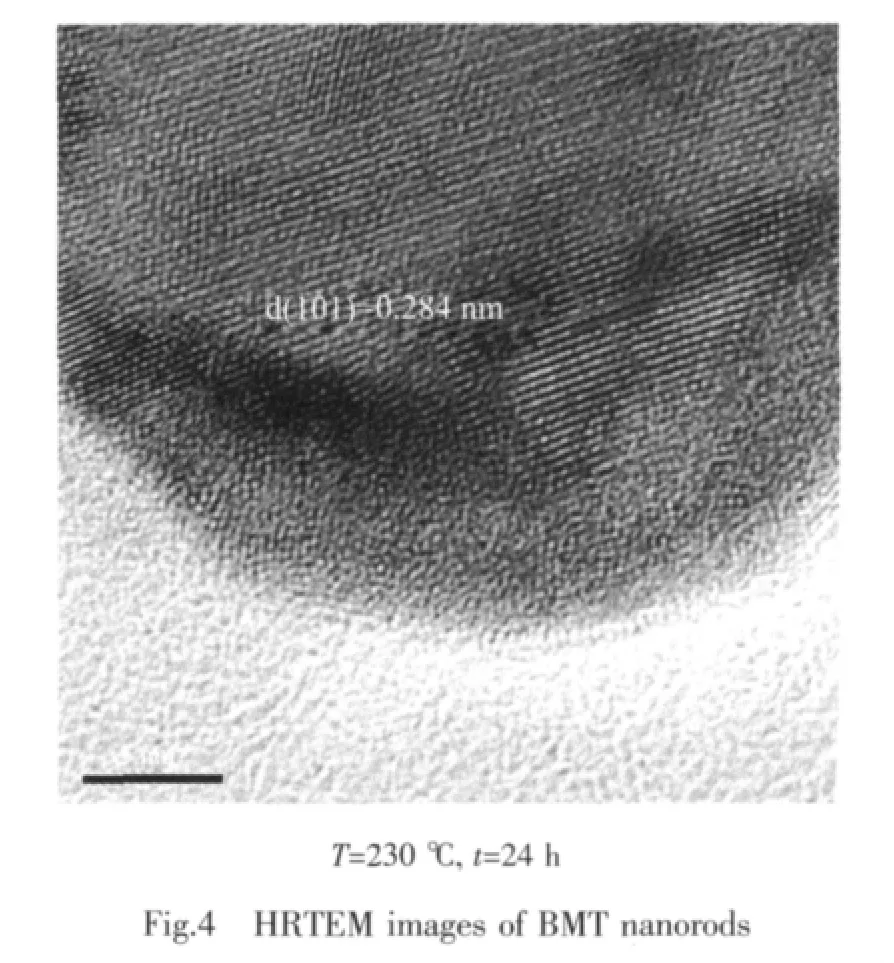
In orderto investigate the detailed crystal structure of as-prepared samples,high-resolution transmission electron microscope(HRTEM)images for BMT nanorods were measured and shown in Fig.4.It reveals the fringes with an interval of 0.28 nm,which is in good agreement with the (101)lattice planes of the perovskite BaTiO3[32].This result might indicate that the low concentration doping of Mg2+ions does not induce the formation of separate purity phases(magnesium metal).

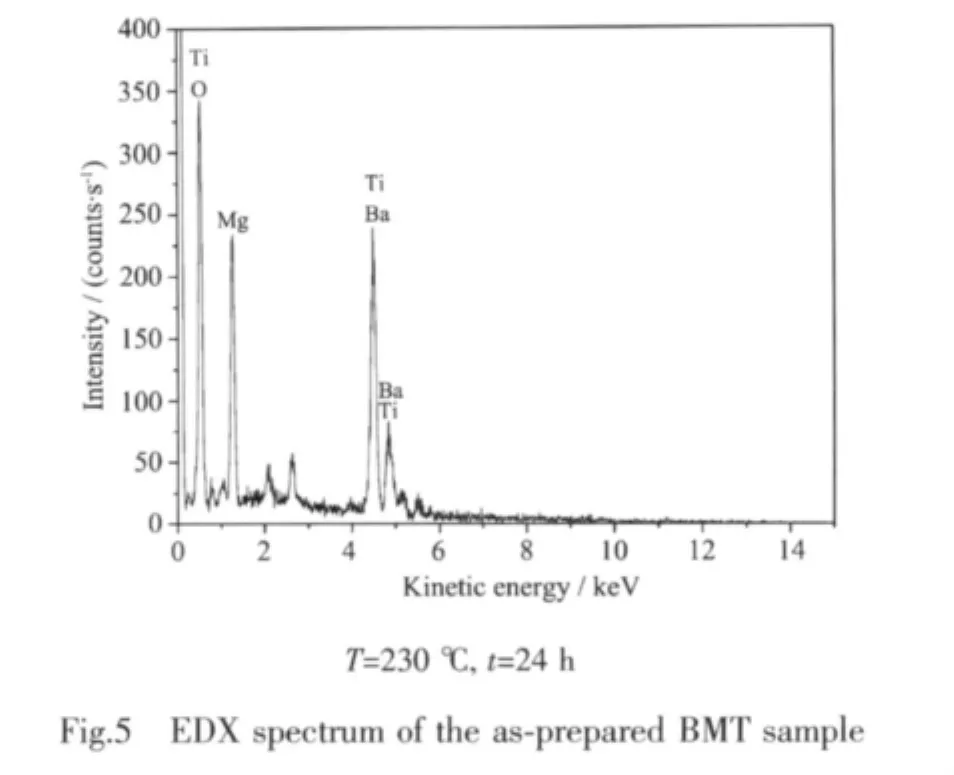
The SEM-EDX analysis reveals that Ba0.96Mg0.40TiO3(BMT)has a homogenous atomic distribution with no other impure elements,as shown in Fig.5.An average atomic ratio of Ba∶Mg∶Ti(0.96∶0.40∶1.00) for Ba0.96Mg0.40TiO3is obtained from measurements at different points.Based on the above results,we can conclude that the resulting materials are of high purity under our preparation conditions.
Fig.6 schematically outlines the possible mechanism involved in the hydrothermal synthesis.Although the crystal growth habit is mainly determined bytheintrinsicstructure,itisalso affected by the external conditions such as pH valueof the solution,saturation,temperature,etc.OH-concentration in the precursorsolution is very important for the microstructure. On the basis of previous report about Na0.5Bi0.5TiO3(NBT)nanostructure and NBT nanoparticles and nanowires[33],at lower concentrations of OH-and for a shorter reaction time,only nanoparticles are obtained.,Primary nanoparticles grow and aggregate with the increase of OH-concentration,when the product is picked up freshly,the formation of nanowires begins.When the OH-concentration is further increased,nanowires are obtained accompanied by the disappearance of the spherical particles. Our experimental results are in accordance with the above mechanism.Thus,OH-concentration (i.e.pH value)plays an important role in the hydrothermal process.

In this work,the condition ofthe alkaline medium as a factor is considered to play a key part in the formation of BMT nanorods.At a lower OH-concentration (OH-concentration:3 mol·L-1),BMT nuclei produced in solution can aggregate to form small particles.These particles may serve as crystal seedstogrow thenanorodsstructure.With the alkaline increase (OH-concentration:8 mol·L-1),a large amount of BMT nuclei produced in the solution leads to formation of the very high supersaturation solution,which favors the formation of rod structure.When OH-concentration is relatively low (OH-concentration:5 mol·L-1),nanorods as well as spherical nanoparticals are obtained because of lower driving force,which come from the lower chemical potential. Hence, the special growth behavior formation of BMT nanorods in the present route is attributed to the highly alkaline medium[34].
2.1.3 Laser Raman spectrum
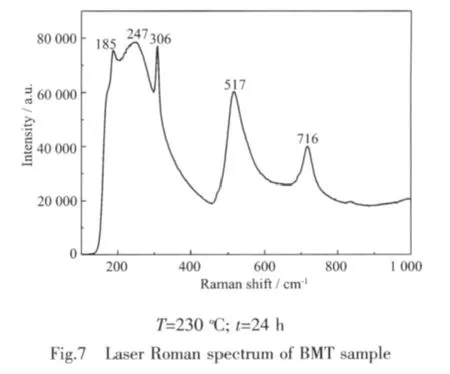
Laser Raman spectrum of BMT sample is shown in Fig.7.Our Raman data on the BMT powder is in agreement with the published results[35].The peak at 716 cm-1is attributed to the symmetric Ti-O stretching vibration,while at 517 cm-1to the asymmetric one;the 306 and 247 cm-1modes are ascribed to the O-Ti-O bendingvibration.TheRaman modesofthe corresponding lower wavenumbers,such as the modes at 185 cm-1,originate mainly from the vibrations between Ba and O atoms.
2.1.4 UV-Vis diffuse reflectance spectra
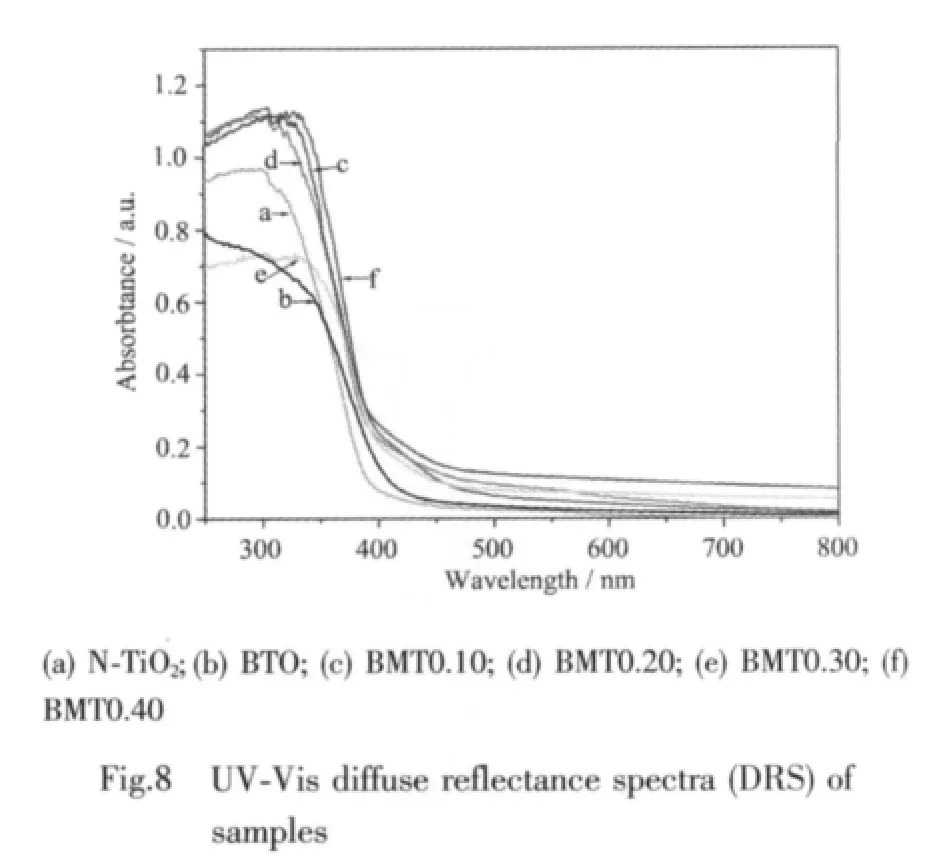
The UV-Vis DRS of the prepared BMT samples are shown in Fig.8.As a comparison,the spectra of N-TiO2and BTO were also measured.The absorption onset wavelength λgof BMT is around 475 nm,which is shifted 20 and 75 nm to visible region compared to BTO and N-TiO2.The band gap energy Egof BMT is calculated to be about 2.61 eV,based on the formula:Eg(eV)=1 239.8/λg(nm)[36],indicating that BMT photocatalysts have a suitable band gap for photocatalytic decomposition of organic contaminants under visible light irradiation.The DRS of BMT photocatalysts have steep shape which indicates that the absorption relevant to the band gap is due to the intrinsic transition of the nanomaterials[37].Furthermore,the absorption edge of BMT0.40 shift red comparedwiththatofBMT0.30,BMT0.20,and BMT0.10,indicating that metal elements doping is one of the typical approaches to extend the spectral response of semiconductor photocatalysts.Thus,the performance of BMT0.40 is better.
2.1.5 Photoluminescence spectrum
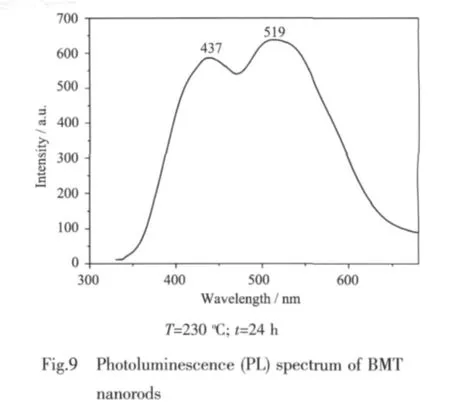
Fig.9 shows photoluminescence(PL)spectrum of BMT nanorods.It can be seen that BMT has obviously luminous phenomena in the range of 350 to 700 nm under UV irradiation of about 325 nm.The asprepared BMT sample shows the presence of two broad PL bands.The first band is at 437 nm(blue emission),while the second is in the range from 500 to 530 nm(green emissions).It has been reported that there are a large number of surface oxygen vacancy in ZnO nanorods due to their large specific surface area[38].It can be deduced that there also exited surface oxygen vacancy in BMT nanorods and the two emission bands might arise respectively from the excitonic emission and surface-defect[38].
2.2 Photocatalytic activities of BMT samples
2.2.1 Degradation of MO using BMTphotocatalysts
Photodegradation experiments of MO were carried out under visible light irradiation at wavelength about420 nm in orderto testthe photocatalytic performance of BMT photocatalysts.For comparison,the photodegradation of MO by N-doped TiO2,BTO and that without any catalyst were also carried out.Temporal course of the photodegradation of MO in different catalyst aqueous dispersions is shown in Fig.10(a)while the corresponding UV-Vis spectral changes of these solutions are displayed in the inset.The results show that MO solution is stable under visible light irradiation in the absence of any catalyst.It also can be observed that N-TiO2exhibits inefficient photocatalytic degradation with MO decomposition rate of40.0% within 360 min irradiation.In contrast,BMT samples reveal highphotocatalytic activitieswith theMO degradation efficiencies of81.0,84.0,90.0,and 93.0%,respectively,as the doping amount of Mg2+increased.Obviously, BMT0.40 exhibits the highest photocatalytic degradation efficiency among those samples.As shown in Fig.10(a),the photodegradation of MO by BMT0.40 is much higher than that of BTO.Temporal course of the photodegradation of MO by BMT samples prepared at different OH-concentrations is shown in Fig.10(b).It can be seen that BMT nanorods prepared at OH-concentration of 8 mol·L-1show relatively higher photocatalytic degradation efficiency.This result shows that the performance of BMT nanorods without any mixed phase is better.

2.2.2 Stability of BMT as the photocatalyst
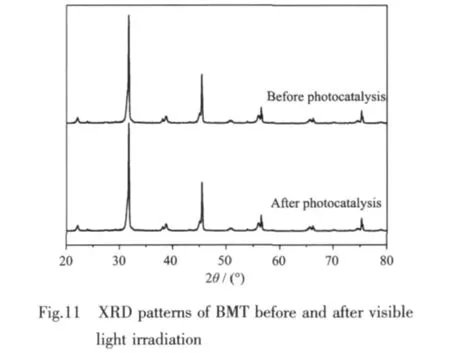
Fig.11 indicates the XRD patterns of the BMT sample before and after 360 min visible light irradiation.Both the position and the intensity of the peaks in the XRD pattern are almost the same as those of BMT before irradiation.As shown in this result,BMT photocatalystis considered to be relatively stable to visible light irradiation under the present experimental conditions.This result indicates a possibility for application of BMT photocatalyst in the waste water treatment.
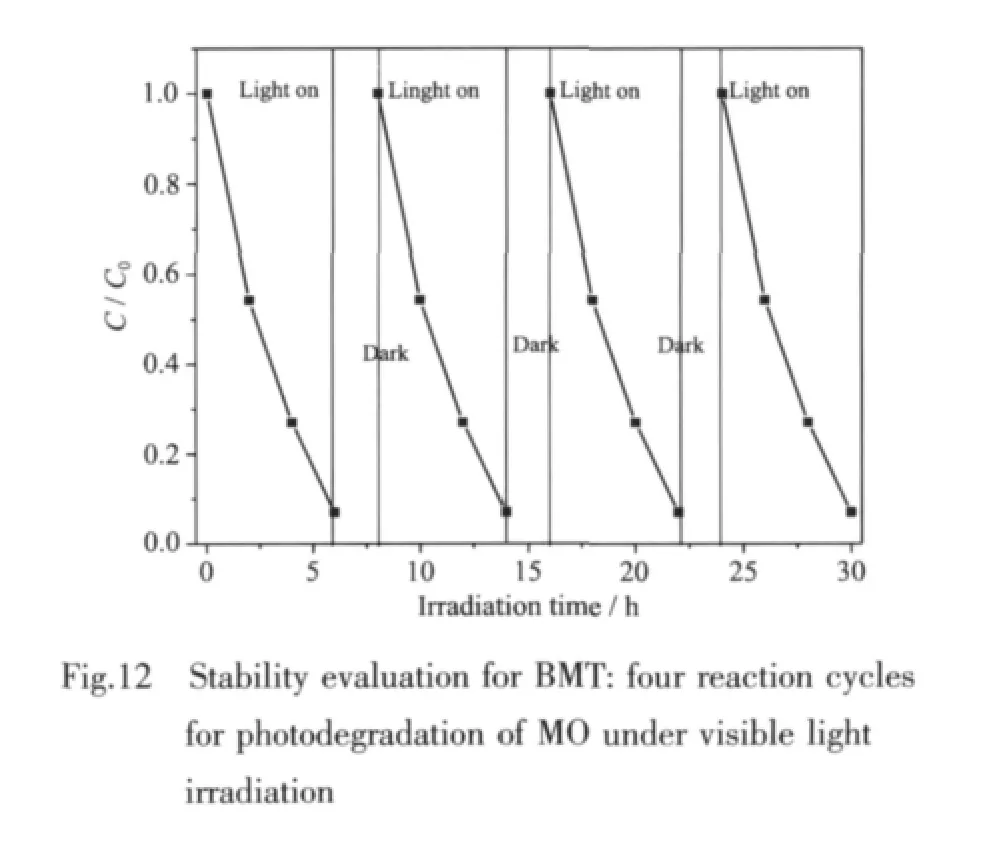
The stability tests were also investigated by carrying out recycling reactions four times for the photodegradation of MO over BMT photocatalyst under visible light irradiation,and the results are shown in Fig.12.No significant decrease in catalytic activity is observed in the recycling reactions.Combined with the XRD patterns,all evidences demonstrate that BMT photocatalyst has good stability.
2.3 Photocatalytic activity mechanism discussion
In a typical photodegradation of organic pollutants process, when the semiconductor is irradiated by light,the photoexcited electrons can be transferred to the conduction band (CB)from the valence band (VB)and whilst the holes form in the VB.Then the photoexcited holes in the VB can form·OH (hydroxyl radical)that can oxidize the organic pollutants and the electrons in the CB participate in the reduction process.Some organic pollutants can be oxidized by TiO2photocatalyst[39]and the degradation products are usually CO2and H2O.Based on the above consideration,we presume that the CB of BMT is composed of Ti3d orbital and VB is formed by the hybridized Ba6s and O2p orbitals,and the degradation products are CO2and H2O.The possible photocatalytic mechanism of BMT is established,and a schematic diagram is shown in Fig.13.
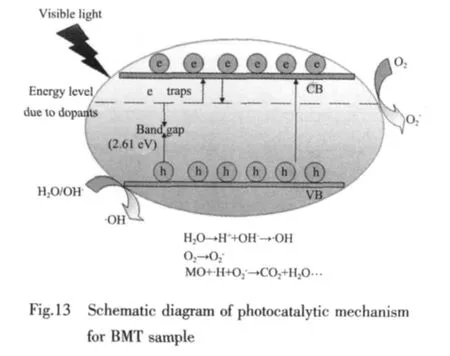
Thus, the photocatalytic activity of the semiconductor is very closely related to its corresponding band structure.The band gap of oxides is generally defined by the O2p level and metal d level[20].The higher photocatalytic activity of BMT over TiO2is attributed to the suitable band gap (2.61 eV)and stable e-h pair formation in the VB formed by the hybrid orbitals of Ba6s and O2p and the CB of Ti3d[18,20].
3 Conclusions
Magnesium doped barium titanate(BMT)nanostructures were synthesized by a facile hydrothermal process withoutthe use ofany surfactants or templates.The optical band gap of BMT is estimated tobeabout2.61 eV,which provesthatBMT photocatalysts can respond to the visible light.Besides,based on the structural analysis of samples obtained at different conditions,we also proposed a possible mechanism to account for the formation of these distinctive morphologies.Most importantly,BMT photocatalysts with good stability exhibithigher photocatalytic performance in the degradation of methyl orange under visible light irradiation than that of N-TiO2.
[1]Ghorai T K,Biswas S K,Pramanik P.Appl.Surf.Sci.,2008,254:7498-7504
[2]SONG Xu-Chun(宋旭春),ZHENG Yi-Fan(鄭遺凡),CAO Guang-Sheng(曹廣勝),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2005,21(12):1897-1900
[3]YANG Ya-Hui(楊亞輝),CHEN Qi-Yuan(陳啟元),YIN Zhou-Lan(尹周瀾),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2007,23(5):771-777
[4]YAN Ya(嚴(yán)亞),Lü Ying(呂瑛),XIA Yi(夏怡),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(10):1999-2004
[5]YU Chang-Lin(余長林),ZHOU Wan-Qin(周晚琴),YU Jimmy C.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(10):2033-2038
[6]LI Yue-Jun(李躍軍),CAO Tie-Ping(曹鐵平),WANG Chang-Hua(王長華),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(10):1975-1980
[7]Wang H Q,Wu Z B,Liu Y,et al.Chemosphere,2008,74:773-778
[8]Lu S Y,Wu D,Wang Q L,et al.Chemosphere,2011,82:1215-1224
[9]Kang S Z,Yang Y K,Bu W B,et al.J.Solid State Chem.,2009,182:2972-2976
[10]Castro A L,Nunes M R,Carvalho M D,et al.J.Solid State Chem.,2009,182:1838-1845
[11]Zhang J W,Jin Z S,Feng C X,et al.J.Solid State Chem.,2011,184:3066-3073
[12]Tian L H,Ye L Q,Deng K J,et al.J.Solid State Chem.,2011,184:1465-1471
[13]Yu J G,Xiong J F,Cheng B,et al.J.Solid State Chem.,2005,178:1968-1972
[14]Xu J J,Chen M D,Fu D G.Appl.Surf.Sci.,2011,257:7381-7386
[15]Su S,Zuo R Z,Lü D Y,et al.Powder Technol.,2012,217:11-15
[16]Xiao C J,Jin C Q,Wang X H.J.Mater.Process.Technol.,2009,209:2033-2037
[17]Yao W F,Xu X H,Wang H.Appl.Catal.B:Environ.,2004,52:109-116
[18]Wang Z Z,Qi Y J,Qi H Y,et al.J.Mater.Sci.:Mater.Electron.,2010,21:523-528
[19]LI Yue-Jun(李躍軍),CAO Tie-Ping(曹鐵平),WANG Chang-Hua(王長華),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2011,27(10):1975-1980
[20]Hou J G,Jiao S Q,Zhu H M,et al.J.Solid State Chem.,2011,184:154-158
[21]Zhou T F,Hu J C.Environ.Sci.Technol.,2010,44:8698-8703
[22]Cheng H F,Huang B B,Dai Y,et al.J.Solid State Chem.,2009,182:2274-2278
[23]Xu J J,Chen M D,Fu D G.Appl.Surf.Sci.,2011,257:7381-7386
[24]Xu J,Wang W Z,Shang M,et al.J.Hazard.Mater.,2011,196:426-430
[25]Hou J G,Wang Z,Jiao S Q,et al.J.Hazard.Mater.,2011,192:1772-1779
[26]Hou J G,Cao R,Jiao S Q,et al.Appl.Catal.B:Environ.,2011,104:399-406
[27]Yu H G,Yu J G,Cheng B.Chemosphere,2007,66:2050-2057
[28]Wang Z Z,Qi Y J,Qi H Y,et al.J Mater Sci:Mater Electron,2010,21:523-528
[29]Hou Y D,Wang X C,Wu L,et al.Chemosphere,2008,72:414-421
[30]Xu Q,Chen S T,Chen W,et al.J.Mater.Sci.,2006,41:6146-6149
[31]Chen W,Yao X,Wei X Y.J.Mater.Sci.,2008,43:1144-1150
[32]Hennings D F K,Metzmacher C,Schreinemacher B S.J.Am.Ceram.Soc.,2001,84(1):179-182
[33]Jiang X P,Lin M,Tu N,et al.J.Alloy.Compd.,2011,509:9346-9350
[34]Yang J H,Zheng J H,Zhai H J,et al.J.Alloy.Compd.,2009,481:628-631
[35]Zhang M S,Yu J,Chen W C,et al.Prog.Cryst.Growth Charact.Mater.,2000,33-42
[36]Liu Z,Xu X X,Fang J Z,et al.Appl.Surf.Sci.,2012,258:3771-3778
[37]Zhu X Q,Zhang J L,Chen F.Chemosphere,2010,78:1350-1355
[38]LI Li(李麗),YANG He-Qing(楊合情),MA Jun-Hu(馬軍虎),et al.Chinese J.Inorg.Chem.(Wuji Huaxue Xuebao),2012,28(1):25-29
[39]LI Ai-Chang(李愛昌),LI Gui-Hua(李桂花),ZHENG Yan(鄭琰),et al.Acta Phys.-Chim.Sin.(Wuli Huaxue Xuebao),2012,28(2):457-464
Hydrothermal Synthesis and Photocatalytic Properties of Nanostructures Magnesium Doped BaTiO3
LIN Xue1,2GUAN Qing-Feng*,1LI Hai-Bo3BA Chun-Hua2WANG Dan-Dan2MENG Fan-Jun2
(1School of Materials Science and Engineering,Jiangsu University,Zhenjiang,Jiangsu 212013,China)
(2College of Chemistry,Key Laboratory of Preparation and Application Environmentally Friendly Materials of the Ministry of Education,Jilin Normal University,Siping,Jilin 136000,China)
(3College of Physics,Jilin Normal University,Siping,Jilin 136000,China)
Magnesium doped barium titanate photocatalysts(Ba(1-x)MgxTiO3(x=0,0.10,0.20,0.30,0.40),BMT)were synthesized by a facile hydrothermal process without the use of any surfactants or templates and characterized by X-ray diffraction (XRD),field emission scanning electron microscope (FESEM),transmission electron microscope(TEM),and UV-Vis diffuse reflectance spectroscopy(DRS).FESEM results show that different morphologies could be fabricated by simply manipulating the concentrations of hydroxide ions.In this case,hydroxide ions seem to play a key role in controlling the formation of seeds and growth rates of BMT particles.On the basis of structural analysis of samples obtained at different conditions,a possible mechanism for the formation of these distinctive morphologies is proposed.UV-Vis DRS results demonstrate that the band gap of BMT sample is about 2.61 eV.The as-prepared BMT photocatalysts exhibite higher photocatalytic activities for the degradation of methyl orange(MO)under visible light irradiation than that of N-TiO2obtained by the traditional method.Furthermore,BMT nanorods prepared at OH-concentration of 8 mol·L-1show the highest photocatalytic activity.A 93.0%degradation of MO solution(0.01 mmol·L-1)is obtained over this catalyst after visible light irradiation for 360 min.In addition,there is no significant decrease in the photocatalytic activity after 4 recycles,indicating that BMT is a stable photocatalyst for degradation of MO under visible light irradiation.
barium titanate;Mg-doping;hydrothermal method;photocatalytic degradation;visible light irradiation
O643
A
1001-4861(2012)10-2248-09
2012-01-20。收修改稿日期:2012-04-24。
吉林省科技創(chuàng)新課題(No.20090144)資助項(xiàng)目。
*通訊聯(lián)系人。E-mail:guanqf@ujs.edu.cn

