急性心肌梗死大鼠缺血心肌中差異microRNA的表達(dá)譜分析*
王海華, 姜玉新, 高 欣, 閔志雪, 林 浩, 崔鳳娟
(皖南醫(yī)學(xué)院 1生理學(xué)教研室, 2醫(yī)學(xué)一系,安徽 蕪湖 241002)
急性心肌梗死大鼠缺血心肌中差異microRNA的表達(dá)譜分析*
王海華1△▲, 姜玉新1▲, 高 欣2, 閔志雪1, 林 浩2, 崔鳳娟1
(皖南醫(yī)學(xué)院1生理學(xué)教研室,2醫(yī)學(xué)一系,安徽 蕪湖 241002)
目的篩選急性心肌梗死(AMI)大鼠缺血心肌中差異表達(dá)的microRNAs(miRNAs),預(yù)測(cè)其靶基因并分析其可能的生物學(xué)功能。方法結(jié)扎冠狀動(dòng)脈左前降支建立雄性Wistar大鼠AMI模型,同時(shí)檢測(cè)其心電圖和頸總動(dòng)脈血壓變化,并用TTC法測(cè)定心肌梗死面積;假手術(shù)(sham)組除不結(jié)扎冠狀動(dòng)脈左前降支外,其它實(shí)驗(yàn)程序與AMI組相同。心肌缺血4 h后取梗死區(qū)心肌組織,提取總RNA進(jìn)行microRNA芯片雜交檢測(cè),并用real-time PCR進(jìn)行驗(yàn)證;生物信息學(xué)方法預(yù)測(cè)差異miRNAs的靶點(diǎn)并分析其生物學(xué)功能。結(jié)果心電圖、血壓檢測(cè)及病理切片證實(shí)AMI模型制備成功。Microarray檢測(cè)結(jié)果表明,與sham組相比,獲得11個(gè)與急性心肌梗死相關(guān)的miRNAs,其中6個(gè)miRNAs上調(diào)表達(dá),5個(gè)miRNAs下調(diào)表達(dá);已知3個(gè)miRNAs(rno-miR-181c、rno-miR-146b和rno-miR-208)參與了心血管功能的調(diào)節(jié),8個(gè)miRNAs(rno-miR-672*、rno-miR-743b、rno-miR-128、rno-miR-138-1*、rno-miR-336、rno-miR-138-2*、rno-miR-325-3p和rno-miR-3572)是否與心血管功能有關(guān)尚不清楚,可能是心肌梗死相關(guān)的新的生物標(biāo)志物。預(yù)測(cè)的miRNA靶基因中的一部分亦與心血管功能相關(guān)。結(jié)論本研究獲得的與AMI相關(guān)的差異miRNAs,可能是急性心肌梗死新的生物標(biāo)志物和潛在的治療靶點(diǎn)。
急性心肌梗死; 微小RNA; 表達(dá)譜; 生物信息學(xué)
冠心病、心肌缺血和心肌梗死,尤其是急性心肌梗死(acute myocardial infarction, AMI)是臨床的常見病和多發(fā)病,其發(fā)病率及致死率逐年升高。MicroRNAs (miRNAs)是一類高度保守的內(nèi)源性非編碼單鏈小分子RNA,長度約為19~25個(gè)核苷酸,對(duì)蛋白表達(dá)起轉(zhuǎn)錄后調(diào)控作用,且其表達(dá)具有一定的組織特異性[1]。已有研究表明,miRNAs參與了心肌肥厚、心肌缺血、心律失常等多種病理生理過程[2-8],是一類重要的內(nèi)源性調(diào)節(jié)因子。miRNAs在AMI的發(fā)生發(fā)展過程中其表達(dá)呈現(xiàn)出時(shí)序性。如miR-1和miR-206在心梗后1周、2周和4周的含量持續(xù)上升,心梗4周時(shí)miR-1上調(diào)20倍和miR-206上調(diào)15倍[5]。van Rooij等[9]亦觀察到此現(xiàn)象,在大鼠心梗后3 d和14 d梗死心肌邊緣區(qū)(border zone)和遠(yuǎn)離區(qū)(remote zone)檢測(cè)到了不同數(shù)量的miRNAs的表達(dá)。這說明miRNAs在心肌梗死的發(fā)病過程具有多樣性與復(fù)雜性。但miRNAs在心肌缺血中的功能與機(jī)制研究尚處于起步階段,尤其是有關(guān)心肌缺血早期miRNAs改變的報(bào)道較少。本研究擬復(fù)制大鼠AMI模型,篩選梗死心肌差異表達(dá)的miRNAs,并通過生物信息學(xué)預(yù)測(cè)其靶基因,探討其與AMI的發(fā)生發(fā)展的關(guān)系,為防治心肌缺血性損傷提供新的實(shí)驗(yàn)依據(jù)和可能的治療靶點(diǎn)。
材 料 和 方 法
1動(dòng)物及分組
雄性清潔級(jí)Wistar大鼠[(200±20) g] 6只,由南京青龍山動(dòng)物繁殖場提供,實(shí)驗(yàn)動(dòng)物合格證號(hào)為SCXK(蘇)2007-0001,隨機(jī)分為2組(n=3):AMI組和假手術(shù)(sham)組。AMI大鼠25%氨基甲酸乙酯(4 mL/kg,ip)麻醉后,仰臥固定于鼠臺(tái)上,記錄心電圖,通過插入氣管插管,連接動(dòng)物呼吸機(jī)維持正常通氣(潮氣量3 mL/kg,呼吸比1.5∶1,呼吸頻率70min-1)。胸骨左側(cè)順第3~4肋肋間隙方向切開皮膚,逐層分離皮下組織、肌肉,開胸器于3~4肋間撐開進(jìn)胸,暴露心臟,用6-0無損傷縫線在左心耳下緣1~2 mm處連同少量心肌組織縫扎左冠狀動(dòng)脈前降支。穩(wěn)定10 min確定無出血后,逐層關(guān)胸。心電圖檢測(cè)可見ST段呈弓背向上型明顯抬高,血壓明顯下降。2, 3, 5-氯化三苯基四氮唑(2, 3, 5-triphenyltetrazolium chloride, TTC)染色法對(duì)冠狀動(dòng)脈左前降支結(jié)扎4 h的大鼠心肌進(jìn)行梗死面積測(cè)定,通過對(duì)比分析證實(shí)AMI模型成功。心肌缺血4 h后取梗死區(qū)心肌組織于液氮保存。Sham組除不結(jié)扎冠狀動(dòng)脈左前降支外,其它處理與AMI組相同。
2缺血區(qū)心肌組織總RNA的提取
將約100 mg缺血區(qū)心肌組織用液氮研磨成粉末,加入1 mL TRIzol?(Invitrogen)混合,按TRIzol和miRNeasy Mini Kit(Qiagen)說明書進(jìn)行總RNA提取,提取的RNA用ND-1000分光光度計(jì)(Nanodrop Technologies)檢測(cè)其質(zhì)量和含量,用瓊脂糖凝膠電泳檢測(cè)RNA的完整性,兩者均合格后方可進(jìn)行后續(xù)實(shí)驗(yàn)。
3芯片雜交
3.1探針標(biāo)記 RNA用miRCURYTMHy3TM/Hy5TMPower Labeling Kit(Exiqon)按說明書進(jìn)行標(biāo)記。用Hy3TM熒光標(biāo)記液進(jìn)行3’末端標(biāo)記1 μg/樣本,并用T4 RNA連接酶按下列步驟進(jìn)行連接:2.0 μL RNA溶液(0.5 g/L)和1.0 μL CIP緩沖液及CIP(Exiqon)混合后37 ℃孵育30 min,95 ℃終止反應(yīng)5 min;然后加入3.0 μL標(biāo)記緩沖液,1.5 μL Hy3TM熒光標(biāo)記液(Exiqon),2.0 μL DMSO,2.0 μL標(biāo)記酶,混勻后16 ℃孵育1 h,65 ℃終止反應(yīng)15 min。
3.2miRNA芯片雜交 雜交用芯片為miRCURYTMLNA Array 18.0(Exiqon,包括miRBase 18.0注釋的覆蓋所有人、大鼠和小鼠的miRNAs及與這些物種相關(guān)的病毒miRNAs,共3 100個(gè)探針),雜交按照說明書進(jìn)行。具體操作如下:取25 μL Hy3TM標(biāo)記的樣本與25 μL雜交緩沖液混合,95℃變性2 min,冰上放置2 min,然后在12-Bay Hybridization System(NimbleGen)上進(jìn)行芯片雜交,56 ℃雜交16~20 h,用Wash Buffer Kit(Exiqon)洗滌芯片3次,400 r/min離心5 min甩干。用Axon GenePix 4000B Microarray Scanner(Axon Instruments)進(jìn)行圖像掃描。為保證結(jié)果的可重復(fù)性和可靠性,每組均用3張芯片進(jìn)行3次獨(dú)立實(shí)驗(yàn)。
3.3芯片結(jié)果的real-time PCR驗(yàn)證
3.3.1引物設(shè)計(jì) 隨機(jī)挑取4個(gè)差異表達(dá)的miRNAs,用Primer 5.0進(jìn)行特異性引物設(shè)計(jì)。同時(shí)以U6為內(nèi)參照,見表1。引物由生工生物工程(上海)股份有限公司合成。

表1 Real-time PCR使用的引物
3.3.2Real-time PCR 反應(yīng)體系為10 μL:5 μL 2×PCR master mix(Exiqon),0.5 μL PCR特異引物F(10 μmol/L),0.5 μL PCR特異引物R(10 μmol/L),2 μL H2O,加入2 μL cDNA后混勻。PCR反應(yīng)條件為:95 ℃ 10 min;95 ℃ 10 s;60 ℃ 60 s(收集熒光),40個(gè)循環(huán)。為建立PCR產(chǎn)物的熔解曲線,擴(kuò)增反應(yīng)結(jié)束后,按 95 ℃ 10 s、60 ℃ 60 s、95 ℃ 15 s,并從60 ℃緩慢加熱到99 ℃(儀器自動(dòng)進(jìn)行-Ramp Rate為2%)。各樣品的目的miRNAs和U6分別進(jìn)行real-time PCR反應(yīng)。PCR反應(yīng)在ABI PRISM7900 System(Applied Biosystems)進(jìn)行。
3.4miRNA靶基因的預(yù)測(cè)和功能分析
3.4.1miRNA靶基因的預(yù)測(cè) 使用miRanda(http://microrna.org/microrna/home.do)、miRBase(http://www.ebi.ac.uk/enright-srv/microcosm/htdocs/targets/v5/)和miRDB(http://mirdb.org/miRDB/)常用數(shù)據(jù)庫對(duì)差異表達(dá)的miRNAs進(jìn)行靶基因預(yù)測(cè)。為了減少假陽性,同一miRNA的靶基因至少在2個(gè)以上的數(shù)據(jù)庫同時(shí)出現(xiàn),方可進(jìn)行后續(xù)分析。
3.4.2miRNA靶基因的功能分類 根據(jù)Gene Ontology(http://www.geneontology.org)的分類原則對(duì)miRNA靶基因進(jìn)行分類,同時(shí)分析了每一大類中的主要亞類(前10個(gè)亞類)。
4數(shù)據(jù)分析
掃描的圖片用GenePix Pro 6.0軟件(Axon)進(jìn)行數(shù)據(jù)提取,并進(jìn)行數(shù)據(jù)標(biāo)準(zhǔn)化處理。差異表達(dá)的miRNAs用volcano plot filtering鑒定,用MeV 4.6(TIGR)進(jìn)行系統(tǒng)聚類。實(shí)時(shí)定量PCR數(shù)據(jù)采用2-ΔΔCt法進(jìn)行分析。芯片雜交及數(shù)據(jù)分析均由上海康成生物工程有限公司完成。數(shù)據(jù)以均數(shù)±標(biāo)準(zhǔn)差(mean±SD)表示,組內(nèi)比較用配對(duì)t檢驗(yàn),多組均數(shù)
比較用單因素方差分析(One-way ANOVA),兩兩比較用SNK法,以P<0.05為差異有統(tǒng)計(jì)學(xué)意義。
結(jié) 果
1各組大鼠心電圖及心肌梗死面積的示意圖
與sham組相比,AMI組大鼠II導(dǎo)聯(lián)心電圖ST段抬高,出現(xiàn)寬大的QRS波,T波倒置,同時(shí)其血壓明顯下降,見圖1。通過TTC法對(duì)冠狀動(dòng)脈左前降支結(jié)扎4 h的大鼠心肌進(jìn)行梗死面積測(cè)定,可明顯見到缺血未梗死區(qū)(紅色區(qū))和缺血梗死區(qū)(白色區(qū)),見圖2,通過對(duì)比分析證實(shí)AMI模型成功。
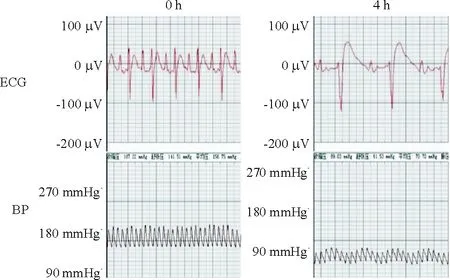
Figure 1. Representative changes of electrocardiogram and blood pressure in rats during acute myocardial infarction.
圖1急性心梗模型大鼠的心電圖和血壓變化

Figure 2. Observation of ischemic myocardium (TTC staining). A: sham; B~D: ischemic regions of myocardium.
圖2大鼠心肌梗死區(qū)示意圖
2差異表達(dá)的miRNAs篩選
與sham組相比,篩選出11個(gè)與急性心肌梗死相關(guān)的差異表達(dá)miRNAs(上調(diào)>1.3倍或下調(diào)<0.76倍),見表2。其中6個(gè)miRNAs表達(dá)上調(diào),分別是rno-miR-672*、rno-miR-743b、rno-miR-128、rno-miR-138-1*、rno-miR-336和rno-miR-138-2*。5個(gè)miRNAs表達(dá)下調(diào),分別是rno-miR-181c、rno-miR-146b、rno-miR-325-3p、rno-miR-208和rno-miR-3572。在火山圖中可見上調(diào)表達(dá)的6個(gè)miRNAs位于圖中右側(cè),而下調(diào)表達(dá)的5個(gè)miRNAs則位于左側(cè),見圖3A。系統(tǒng)聚類圖中可見差異表達(dá)的miRNAs的變化情況,見圖3B。
表2急性心肌梗死相關(guān)的差異表達(dá)的miRNAs
Table 2. Differentially expressed miRNAs in ischemic myocardium of rats after acute myocardial infarction

miRNAFoldchangePUp-regulationrno-miR-672*2.6431070.043074rno-miR-743b2.0781840.008223rno-miR-1281.5927020.040501rno-miR-138-1*1.4706660.045936rno-miR-3362.9711930.003887rno-miR-138-2*1.6222890.033825Down-regulationrno-miR-181c0.5717890.004967rno-miR-146b0.5414320.023792rno-miR-325-3p0.4381130.036996rno-miR-2080.7355030.031335rno-miR-35720.3908910.048627

Figure 3. Volcano plot (A) and hierarchical clustering (B) of differentially expressed miRNAs.
圖3差異表達(dá)的miRNAs的火山圖和系統(tǒng)聚類分析
3Real-timePCR檢測(cè)
隨機(jī)選取4個(gè)miRNAs(rno-miR-743b、rno-miR-336、rno-miR-146b和rno-miR-181c)進(jìn)行real time-PCR 驗(yàn)證,同時(shí)以U6為內(nèi)參照,對(duì)數(shù)據(jù)進(jìn)行歸一化處理,并與miRNA芯片數(shù)據(jù)進(jìn)行比較發(fā)現(xiàn),兩者的結(jié)果相一致,見圖4,說明miRNA芯片結(jié)果的可靠性。
4miRNAs的功能分析
11個(gè)差異表達(dá)的miRNAs中,已報(bào)道的與心血管系統(tǒng)功能有關(guān)的有3個(gè),分別是rno-miR-181c、rno-miR-146b 和rno-miR-208,見表3。其它的miRNAs在心血管中的功能尚不清楚。
5miRNAs靶基因的預(yù)測(cè)
通過miRanda、miRBase和miRDB 3個(gè)預(yù)測(cè)大鼠miRNA靶點(diǎn)的數(shù)據(jù)庫對(duì)11個(gè)差異miRNAs的靶點(diǎn)進(jìn)行預(yù)測(cè),這些靶基因在3個(gè)數(shù)據(jù)庫中分布的數(shù)量及重疊情況見圖5。由圖可見,在3個(gè)數(shù)據(jù)庫中均出現(xiàn)的共有61個(gè),它們的具體靶點(diǎn)見表4。其它靶基因僅出現(xiàn)在1個(gè)或2個(gè)數(shù)據(jù)庫中(結(jié)果未顯示)。從表4中可見,僅4種miRNAs的靶基因在3個(gè)數(shù)據(jù)庫中均出現(xiàn),這些miRNAs分別是rno-miR-181c、rno-miR-208、rno-miR-336和rno-miR-743b。
對(duì)所有靶基因進(jìn)行GO分類后,對(duì)生物過程(biological process, BP)、細(xì)胞組分(cellular component, CC)和分子功能(molecular function, MF)的前10亞類進(jìn)行了初步分析,結(jié)果表明在BP大類中,參與細(xì)胞過程(cellular process)的靶基因最多,為543個(gè);其次是代謝過程(metabolic process,411個(gè))、主要代謝過程(primary metabolic process,369個(gè))和細(xì)胞代謝過程(cellular metabolic process,350個(gè)),見圖6A;在CC大類中,胞內(nèi)組分(intracellular)的靶基因最多,為520個(gè),其次分別是胞內(nèi)部分(intracellular part,513個(gè))、細(xì)胞器(organelle,439個(gè))和胞內(nèi)細(xì)胞器(intracellular organelle,437個(gè)),見圖6B;而在MF大類中,這些靶基因主要參與了結(jié)合(binding,582個(gè))、蛋白結(jié)合(protein binding,460個(gè))、催化活性(catalytic activity,282個(gè))和水解酶活性(hydrolase activity,113個(gè))等功能,見圖6C。這些靶基因的分布展示了在急性心肌梗死發(fā)生過程中的基因表達(dá)變化。

Figure 4. Confirmation of differentially expressed miRNAs in rat ischemic myocardium by real-time PCR.A:rno-miR-743b;B:rno-miR-336;C:rno-miR-146b;D:rno-miR-181c. Meam±SD.n=3.*P<0.05vssham group.
圖4差異表達(dá)的miRNAs的real-timePCR驗(yàn)證

表3 差異表達(dá)的miRNAs在心血管系統(tǒng)中的功能分析

Figure 5. Distribution of the target genes of differentially expressed miRNAs in ischemic myocardium from AMI rats in three databases, including miRanda, miRBase and miRDB.
圖5miRNA靶點(diǎn)在miRanda、miRBase和miRDB3個(gè)數(shù)據(jù)庫中的分布
表4在miRanda、miRBase和miRDB3個(gè)數(shù)據(jù)庫中均出現(xiàn)的miRNA靶點(diǎn)列表
Table 4. Target genes of differentially expressed miRNAs from AMI rats listed in all three databases, including miRanda, miRBase and miRDB

miRNATargetrno-mir-181cACSL1,BTNL8,CBX7,CD4,CMTM8,CYR61,DNAJA1,DNAJA4,EHD4,EIF4A2,ESM1,HPGD,NCBP1,NNT,OAT,PDAP1,PI4K2B,PLEKHA3,PRKCD,PSIP1,RECQL,SYNPR,TIMP3,TINAG,TMEM27,TN-FRSF11B,VRK3rno-miR-208SLC39A3,SRRrno-miR-336EBP,EIF2AK3,ITIH4,LRRC33,RBMS1,TIMM23rno-miR-743bACCN5,ALG5,AMIGO2,BAG3,BBS7,BCAT1,BTRC,CAPN7,COPB1,CXCL1,DMGDH,ELAVL2,ERRFI1,EXOSC9,HSD3B1,LOC171573,MAPK9,MMP10,PTF1A,RGS14,SLC39A9,SMAD2,SUB1,TANC1,TM7SF3,TTC5
討 論
急性心肌梗死是臨床的常見病和多發(fā)病,其發(fā)病率逐年升高。miRNAs參與急性心肌梗死的病理生理過程[5-6,10],并受到高度關(guān)注。miRNAs在急性心肌梗死的發(fā)生發(fā)展過程中其表達(dá)呈現(xiàn)時(shí)序性。van Rooij等[9]用miRNA芯片分析了在冠狀動(dòng)脈左前降支結(jié)扎所致大鼠心肌梗死模型中梗死心肌邊緣區(qū)和遠(yuǎn)離區(qū)的miRNAs表達(dá),發(fā)現(xiàn)在心梗后第3天,邊緣區(qū)有17個(gè)miRNAs上調(diào),遠(yuǎn)離區(qū)有12個(gè)miRNAs上調(diào);而心梗后第14天則分別變?yōu)?9個(gè)和40個(gè)上調(diào)。本研究成功制備了AMI大鼠模型,并觀察了心梗后4 h的miRNAs表達(dá)譜,共獲得11個(gè)差異表達(dá)的miRNAs。其中有3個(gè)miRNAs(rno-miR-181c、rno-miR-146b和rno-miR-208)參與了心血管功能的調(diào)節(jié)。如miR-208是心梗特有的miRNA,可作為心肌損傷的分子標(biāo)志物[19],在心梗病人中表達(dá)上調(diào)[18]。而我們發(fā)現(xiàn)rno-miR-208在心梗后4 h表達(dá)下調(diào),該結(jié)果說明了miRNA在心梗的發(fā)生發(fā)展過程中呈現(xiàn)動(dòng)態(tài)變化。
miRNAs是一類對(duì)蛋白表達(dá)具有轉(zhuǎn)錄后調(diào)控作用的內(nèi)源性非編碼單鏈小分子RNA,分析心肌梗死相關(guān)的差異表達(dá)miRNAs對(duì)了解其功能非常重要。我們將獲得的11個(gè)差異miRNAs對(duì)miRanda、miRBase和miRDB 3個(gè)數(shù)據(jù)庫進(jìn)行搜索后,獲得了共9 037個(gè)miRNA靶基因,通過對(duì)部分靶基因進(jìn)行功能搜索后發(fā)現(xiàn),它們中有一些參與了心血管功能的調(diào)節(jié),如rno-miR-743b 的靶基因之一CXCL1在白細(xì)胞介素1刺激的心臟成纖維細(xì)胞中高表達(dá)[21],而心臟成纖維細(xì)胞在缺血心肌修復(fù)中具有重要的作用[22-23]。而本研究獲得的幾個(gè)miRNAs及其靶基因(數(shù)據(jù)未顯示)與心血管功能尤其是在AMI的相關(guān)性均未見報(bào)道,如rno-miR-672*、rno-miR-128、rno-miR-138-1*、rno-miR-336、rno-miR-138-2*、rno-miR-325-3p和rno-miR-3572,它們可能是AMI發(fā)生發(fā)展的早期的新的分子標(biāo)志物,且其功能有待進(jìn)一步研究。同時(shí)對(duì)所有靶基因進(jìn)行了GO分類,這些靶基因主要參與了細(xì)胞過程、代謝過程和生物調(diào)節(jié)等生物過程,主要由胞內(nèi)組分、胞內(nèi)細(xì)胞器和膜結(jié)合組分等細(xì)胞組分構(gòu)成,而其功能涉及到蛋白結(jié)合、催化和水解酶活性等,這從總體上反映了AMI過程中miRNAs及其調(diào)控的基因網(wǎng)絡(luò)的變化。
總之,本研究通過對(duì)AMI大鼠的缺血早期的心肌進(jìn)行miRNA芯片篩選,獲得了11個(gè)差異表達(dá)miRNAs,除了已報(bào)道的參與了心血管功能的部分miRNAs外,我們還發(fā)現(xiàn)了幾個(gè)新的與AMI有關(guān)的miRNAs。但這些miRNAs的靶點(diǎn)是什么,它們?cè)谛募∪毖獱顟B(tài)下是如何影響靶蛋白的表達(dá),繼而影響缺血心肌的結(jié)構(gòu)和功能,我們將在細(xì)胞分子水平進(jìn)一步闡明它們?cè)谛募∪毖械淖饔眉皺C(jī)制,為防治和減輕心肌缺血性損傷提供更多的實(shí)驗(yàn)依據(jù)。

Figure 6. Distribution of the target genes of differentially expressed miRNAs in ischemic myocardium from AMI rats using Gene Ontology method. A:biological process;B:cellular component;C:molecular function.
圖6miRNA靶基因的GO分類
[1] Lagos-Quintana M, Rauhut R, Yalcin A, et al. Identification of tissue-specific microRNAs from mouse [J]. Curr Biol, 2002, 12(9): 735-739.
[2] van Rooij E, Sutherland LB, Liu N, et al. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure [J]. Proc Natl Acad Sci U S A, 2006, 103(48): 18255-18260.
[3] van Rooij E, Sutherland LB, Qi X, et al. Control of stress-dependent cardiac growth and gene expression by a microRNA [J]. Science, 2007, 316(5824): 575-579.
[4] Zhao Y, Ransom JF, Li A, et al. Dysregulation of cardiogenesis, cardiac conduction, and cell cycle in mice lacking miRNA-1-2 [J]. Cell, 2007, 129(2): 303-317.
[5] Shan ZX, Lin QX, Fu YH, et al. Upregulated expression of miR-1/miR-206 in a rat model of myocardial infarction [J]. Biochem Biophys Res Commun, 2009, 381(4): 597-601.
[6] Shi B, Guo Y, Wang J, et al. Altered expression of microRNAs in the myocardium of rats with acute myocardial infarction [J]. BMC Cardiovasc Disord, 2010, 10: 11.
[7] 魏 聰,胡 兵,申 鍔. MicroRNAs 在心臟發(fā)育和疾病中的作用[J].中國病理生理雜志,2011,27(3): 611-615.
[8] 唐 艷,王夢(mèng)洪. microRNA-21 轉(zhuǎn)染的心肌細(xì)胞移植對(duì)低溫條件下心力衰竭大鼠的影響[J].中國病理生理雜志,2013,29(1): 1-8.
[9] van Rooij E, Sutherland LB, Thatcher JE, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis [J]. Proc Natl Acad Sci U S A, 2008, 105(35): 13027-13032.
[10] 王 玨,黃偉聰,鄭亮承,等. MicroRNA-24 對(duì)心肌梗死后心肌細(xì)胞凋亡的調(diào)控作用[J].中國病理生理雜志,2013,29(4): 590-596.
[11] Seeger T, Haffez F, Fischer A, et al. Immunosenescence-associated microRNAs in age and heart failure [J]. Eur J Heart Fail, 2013,15(4): 385-393.
[12] Vogel B, Keller A, Frese KS, et al. Refining diagnostic microRNA signatures by Whole-miRNome kinetic analysis in acute myocardial infarction [J]. Clin Chem, 2013, 59(2): 410-418.
[13] Das S, Ferlito M, Kent OA, et al. Nuclear miRNA regulates the mitochondrial genome in the heart [J]. Circ Res, 2012, 110(12): 1596-1603.
[14] Mishra PK, Metreveli N, Tyagi SC. MMP-9 gene ablation and TIMP-4 mitigate PAR-1-mediated cardiomyocyte dysfunction: a plausible role of dicer and miRNA [J]. Cell Biochem Biophys, 2010, 57(2-3): 67-76.
[15] Barjaktarovic Z, Anastasov N, Azimzadeh O, et al. Integrative proteomic and microRNA analysis of primary human coronary artery endothelial cells exposed to low-dose gamma radiation [J]. Radiat Environ Biophys, 2013, 52(1): 87-98.
[16] Jiang X, Ning Q, Wang J. Angiotensin II induced differentially expressed microRNAs in adult rat cardiac fibroblasts [J]. J Physiol Sci, 2013, 63(1): 31-38.
[17] Zile MR, Mehurg SM, Arroyo JE, et al. Relationship between the temporal profile of plasma microRNA and left ventricular remodeling in patients after myocardial infarction [J]. Circ Cardiovasc Genet, 2011, 4(6): 614-619.
[18] Bostjancic E, Zidar N, Stajer D, et al. MicroRNAs miR-1, miR-133a, miR-133b and miR-208 are dysregulated in human myocardial infarction [J]. Cardiology, 2010, 115(3): 163-169.
[19] Ji X, Takahashi R, Hiura Y, et al. Plasma miR-208 as a biomarker of myocardial injury [J]. Clin Chem, 2009, 55(11): 1944-1949.
[20] Xu CC, Han WQ, Xiao B, et al. Differential expression of microRNAs in the aorta of spontaneously hypertensive rats [J]. Sheng Li Xue Bao, 2008, 60(4): 553-560.
[21] Turner NA, Das A, O’Regan DJ, et al. Human cardiac fibroblasts express ICAM-1, E-selectin and CXC chemokines in response to proinflammatory cytokine stimulation [J]. Int J Biochem Cell Biol, 2011, 43(10): 1450- 1458.
[22] Porter KE, Turner NA. Cardiac fibroblasts: at the heart of myocardial remodeling [J]. Pharmacol Ther, 2009, 123(2): 255-278.
[23] Turner NA. Therapeutic regulation of cardiac fibroblast function: targeting stress-activated protein kinase pathways [J]. Future Cardiol, 2011, 7(5): 673-691.
MicroRNAprofileanalysisofischemicmyocardialtissuesfromratswithacutemyocardialinfarction
WANG Hai-hua1, JIANG Yu-xin1, GAO Xin2, MIN Zhi-xue1, LIN Hao2, CUI Feng-juan1
(1DepartmentofPhysiology,2theFirstDepartmentofClinicalMedicine,WannanMedicalCollege,Wuhu241002,China.E-mail:wanghaihua9972@sina.com)
AIM: To identify differentially expressed microRNAs (miRNAs) in ischemic myocardial tissues from the rats with acute myocardial infarction (AMI) by miRNA array technique, and to predict their targets and analyze their functions using bioinformatics.METHODSThe rat models of AMI (n=3) were prepared by ligaturing the left anterior descending coronary artery (LAD) of Wistar rats. Electrocardiogram and blood pressure were detected during the operation, and the myocardial infarct size was measured by 2, 3, 5-triphenyltetrazolium chloride (TTC) staining. Ischemic myocardial tissues were isolated from the infarct area 4 h after ischemia. The same procedure in sham group (n=3) was performed except for ligaturing LAD. Total RNA was extracted from ischemic and normal myocardial tissues. miRNA was isolated from total RNA, labeled with Cy3 and hybridized on miRNA array. Real-time PCR was applied to verify the reliability of miRNA array results. The targets of differentially expressed miRNAs were predicted and their functions were analyzed by bioinformatics.RESULTSRat model of AMI was successfully prepared and verified by electrocardiogram detection, blood pressure measurement and pathological observation. Compared with sham group, microarray screening showed that total 11 AMI-related miRNAs were selected, including 6 up-regulated and 5 down-regulated. Three of them (rno-miR-181c, rno-miR-146b and rno-miR-208) were related to the cardiovascular functions, while the functions of the others (rno-miR-672*, rno-miR-743b, rno-miR-128, rno-miR-138-1*, rno-miR-336, rno-miR-138-2*, rno-miR-325-3p and rno-miR-3572) were unknown and might be novel AMI-related biomarkers. Parts of the miRNA targets were also related to the cardiovascular functions.CONCLUSIONDifferentially expressed miRNAs in AMI rats may serve as novel biomarkers for diagnosis of AMI and potential targets for treatment of AMI.
Acute myocardial infarction; MicroRNA; Expression profiles; Bioinformatics
R363
A
1000- 4718(2013)09- 1546- 08
2013- 05- 09
2013- 07- 12
國家自然科學(xué)基金資助項(xiàng)目(No.81172790);安徽省自然科學(xué)研究重點(diǎn)項(xiàng)目(No.KJ2013A251);皖南醫(yī)學(xué)院重點(diǎn)科研項(xiàng)目培育基金資助項(xiàng)目(No.WK2012Z01)
△通訊作者 Tel: 0553-3932473; E-mail: wanghaihua9972@sina.com
▲并列第1作者
10.3969/j.issn.1000- 4718.2013.09.002
- 中國病理生理雜志的其它文章
- Nrf2-ARE通路在缺氧/吡那地爾后處理減輕大鼠心肌細(xì)胞缺氧復(fù)氧損傷中的作用*
- TNF-α增強(qiáng)人臍帶間充質(zhì)干細(xì)胞條件培養(yǎng)基的體外造血支持功能*
- 抗組胺治療對(duì)實(shí)驗(yàn)性肝炎大鼠肥大細(xì)胞浸潤及c-Kit和SCF表達(dá)的影響*
- Nrf2過表達(dá)對(duì)乙醇刺激下肝星狀細(xì)胞激活與增殖及合成I型膠原的影響*
- 缺氧預(yù)處理減輕內(nèi)質(zhì)網(wǎng)應(yīng)激所致的大鼠心肌微血管內(nèi)皮細(xì)胞損傷*
- 胰島素對(duì)嚴(yán)重?zé)齻缙诖笫笮募⊙趸瘧?yīng)激的影響*

