聯合使用miR-34a及miR-let7對胰腺癌細胞生物學特性的影響
劉宇亭 沈祥國 蘇長青 孫斌 李兆申 徐燦
200433 上海,第二軍醫大學長海醫院消化內科(劉宇亭、沈祥國、李兆申、徐燦);第二軍醫大學東方肝膽外科醫院分子腫瘤研究室(蘇長青、孫斌);東部戰區空軍機關醫院(劉宇亭)
?
·論著·
聯合使用miR-34a及miR-let7對胰腺癌細胞生物學特性的影響
劉宇亭沈祥國蘇長青孫斌李兆申徐燦
200433上海,第二軍醫大學長海醫院消化內科(劉宇亭、沈祥國、李兆申、徐燦);第二軍醫大學東方肝膽外科醫院分子腫瘤研究室(蘇長青、孫斌);東部戰區空軍機關醫院(劉宇亭)
【摘要】目的探討聯合使用兩種具有抑癌作用的microRNA(miRNA)同時轉染人胰腺癌細胞對其生物學特性的影響。方法采用脂質體法將miR-34a及miR-let7單獨或同時轉染胰腺癌PANC1、SW1990細胞及正常胰腺腺泡AR42J細胞,以轉染陰性對照miRNA(miR-NC)組及未轉染組作為對照。應用qRT-PCR法檢測各組細胞miR-34a及miR-let7的表達,MTT法檢測細胞增殖,Transwell小室檢測細胞遷移和侵襲能力,流式細胞儀檢測細胞凋亡。結果MiR-34a轉染組、miR-let7轉染組、雙轉染組細胞的miR-34a、miR-let7表達水平均較miR-NC轉染組及未轉染組顯著上調,差異有統計學意義(P值均<0.05),表明miRNA成功轉染了細胞。雙轉染組的PANC1、SW1990細胞增殖活性分別為(0.665±0.010)%、(0.638±0.030)%,較miR-NC轉染組的(0.974±0.030)%、(0.971±0.050)%,miR-let7轉染組的(0.888±0.050)%、(0.863±0.060)%顯著被抑制,差異有統計學意義(P值均<0.05),較miR-34a轉染組的(0.795±0.060)%、(0.793±0.060)%下降,但差異無統計學意義。AR42J細胞的增殖活性無顯著變化,差異無統計學意義。PANC1細胞miR-34a轉染組、miR-let7轉染組的穿膜細胞數分別為(103.70±3.28)、(100.70±1.76)個/200倍視野,較miR-NC轉染組的(231.30±2.60)個/200倍視野及未轉染組的(153.70±2.60)個/200倍視野顯著減少,雙轉染組穿膜細胞數為(61.67±3.18)個/200倍視野,又較兩個單轉染組顯著減少,差異均有統計學意義(P值均<0.01)。細胞遷移實驗與侵襲實驗的結果一致。SW1990細胞侵襲及遷移能力的變化與PANC1一致。PANC1細胞的miR-34a轉染組、miR-let7轉染組、雙轉染組、miR-NC轉染組、未轉染組的細胞凋亡率分別為(16.66±1.27)%、(15.46±0.33)%、(23.35±1.80)%、(9.33±0.31)%、(8.83±0.36)%。兩單轉染組的細胞凋亡率較miR-NC轉染組、未轉染組顯著增加;雙轉染組又較miR-let7組顯著增加,差異有統計學意義(P值均<0.05),而雙轉染組與miR-34a轉染組的差異無統計學意義。結論雙轉染的胰腺癌細胞增殖活性、細胞遷移和侵襲力均較單轉染細胞顯著下降,而細胞凋亡率較單轉染細胞顯著增加,雙轉染能夠發揮更加顯著的協同抗腫瘤作用。
【關鍵詞】胰腺腫瘤;細胞系,腫瘤;miR-34a;miR-let7;轉染;分子生物學
胰腺癌惡性程度高,手術切除率低,預后差,總體5年生存率不到5%[1]。腫瘤基因治療及免疫治療的出現為人們攻克胰腺癌提供了新的思路和視野。microRNA(miRNA)是一種高度保守的短鏈非編碼RNA,能夠在轉錄后水平調節基因的表達。到目前為止,已有超過1800余種miRNA被陸續報道[2-4],且大量的研究已經證實miRNA的異常表達與腫瘤的發生相關[5],從而為腫瘤的治療提供了新的方向。有研究報道,miR-34a的靶基因在調節細胞凋亡、細胞周期阻滯、DNA修復、血管生成等方面有非常重要的作用,在胰腺癌中表達缺失或者明顯下調,發揮抑癌基因的作用[6-7]。miR-let7存在于正常胰腺腺泡細胞,在低分化胰腺癌組織中表達缺失,提高胰腺癌細胞株miR-let7的表達水平可顯著抑制癌細胞增殖[8]。因此本研究聯合使用兩種miRNA轉染胰腺癌細胞,觀察其對胰腺癌細胞增殖的影響,為胰腺癌的治療提供新的思路。
材料與方法
一、材料
人胰腺癌細胞系PANC1、SW1990細胞及正常胰腺腺泡細胞AR42J由第二軍醫大學附屬長海醫院消化內科實驗室提供。高糖DMEM培養基和胎牛血清購自美國Gibico公司,miR-34a、miR-let7 mimic及陰性對照miRNA (miR-NC)由廣東銳博技術有限公司合成,LipofectamineTM2000試劑和RNA抽提試劑Trizol購自美國Invitrogen公司,實時熒光定量PCR試劑盒購自Applied Biosystems公司,MTT試劑盒購自美國Sigama公司,Transwell小室購自美國Millipore公司,聚碳酸酯膜基質膠和細胞凋亡檢測試劑盒購自美國BD公司。
二、方法
1.細胞培養及miRNA轉染:胰腺癌PANC1細胞及SW1990細胞由高糖DMEM培養基(含10%FBS)培養,正常胰腺腺泡細胞AR42J由1640(含10% FBS)培養基培養,取對數生長期細胞接種于培養板內。實驗分5組:miR-34a轉染組、miR-let7轉染組、miR-34a和miR-let7雙轉染組、miR-NC轉染組及未轉染對照組,每組設6個復孔,按miRNA mimics及LipofectamineTM2000試劑說明書操作。轉染后用無血清培養基饑餓處理細胞4 h,更換含10%FBS的DMEM培養液繼續培養24 h進行下一步實驗。
2.qRT-PCR法檢測細胞miRNA表達:取上述各組轉染24 h細胞,應用Trizol收集細胞總RNA,在紫外分光光度儀上檢測RNA濃度及純度后采用逆轉錄試劑盒反轉錄獲得cDNA,采用實時熒光定量PCR試劑盒進行擴增,以U6為內參。PCR反應條件:95℃ 10 min,95℃ 15 s、60℃ 30 s、72℃ 30 s,40個循環。所有反應設3個復孔。由儀器自帶軟件獲取Ct值,以公式2-ΔΔCt計算mRNA相對表達量。
3.MTT法檢測細胞增殖活性:取上述各組轉染24 h細胞,加MTT 20 μl繼續培養4 h,加DMSO 150 ml終止反應,上酶標儀檢測490 nm處吸光度值(A490值),以單加培養液的孔調零。細胞增殖率=實驗組A490值/對照組A490值×100%。實驗重復3次,取均值。
4.Transwell小室檢測細胞的遷移、侵襲力:預先將基質膠與DMEM 培養液按1∶5混合稀釋后,以100 μl/室鋪入小室隔膜上,37℃過夜風干、凝固。取上述各組轉染細胞,上室加4×104個細胞,容積200 μl。下室加500 μl含20% FBS的DMEM培養液,培養24 h后用結晶紫染色20~30 min,PBS洗滌后用棉簽輕輕擦拭小室內未穿膜細胞,顯微鏡下取3個200倍視野,計數穿膜細胞數。實驗重復3次,取均值。參考駱廣濤等[9]方法,檢測細胞遷移能力,Transwell小室隔膜不鋪基質膠。
5.流式細胞儀檢測細胞凋亡:取對數生長期各組轉染細胞,用不含EDTA的胰酶消化,收集細胞后用預冷PBS洗兩遍,1 500 r/min離心10 min,用Binding Buffer重懸,置冰上加入Annexin V輕混勻后加入PI染色,上流式細胞儀測細胞凋亡。實驗重復3次。
三、統計學處理
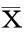
結果
一、MiRNA轉染細胞的鑒定
PANC1、SW1990、AR42J細胞轉染miRNA后,miR-34a轉染組、雙轉染組細胞的miR-34a表達水平均較miR-let7轉染組、miR-NC轉染組及未轉染組顯著上調;miR-let7轉染組、雙轉染組細胞的miR-let7表達水平均較miR-34a轉染組、miR-NC轉染組、未轉染組顯著上調,差異均有統計學意義(P值均<0.05)。見表1、2。表明miRNA成功轉染了細胞。
二、各組轉染細胞增殖的變化
雙轉染組的PANC1、SW1990細胞增殖活性較miR-NC轉染組、miR-let7轉染組顯著被抑制,差異均有統計學意義(P值均<0.05),而與miR-34a轉染組的差異無統計學意義。AR42J細胞各轉染組間的增殖活性無顯著變化,差異無統計學意義(表3)。

表1 3種細胞系中各轉染組miR-34a的相對表達量

表2 3種細胞系中各轉染組miR-let7的相對表達量
三、各組轉染細胞遷移和侵襲力的變化
胰腺癌PANC1細胞及SW1990細胞的miR-34a轉染組及miR-let7轉染組的穿膜細胞數較miR-NC轉染組及未轉染組顯著減少,雙轉染組的穿膜細胞數又較兩個單轉染組顯著減少,差異均有統計學意義(P值均<0.01)。見圖1、2,表4、5。提示同時轉染miR-34a及miR-let7能夠更顯著地抑制胰腺癌細胞的侵襲及遷移能力。
四、各組轉染細胞的凋亡變化
胰腺癌PANC1細胞的miR-34a轉染組、miR-let7轉染組、雙轉染組、miR-NC轉染組、未轉染組的細胞凋亡率分別為(16.66±1.27)%、(15.46±0.33)%、(23.35±1.80)%、(9.33±0.31)%、(8.83±0.36)%(圖3)。miR-34a轉染組、miR-let7轉染組的細胞凋亡率較miR-NC轉染組、未轉染組顯著增加;雙轉染組又較miR-let7轉染組顯著增加,差異有統計學意義(P值均<0.05),而與miR-34a轉染組的差異無統計學意義,提示雙轉染兩種miRNA能進一步提高胰腺癌細胞的凋亡率。
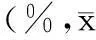
表3 3種細胞系中各轉染組細胞增殖活性±s)
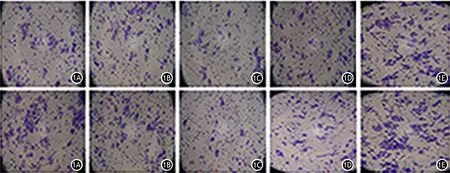
圖1 miR-34a轉染組(1A)、miR-let7轉染組(1B)、雙轉染組(1C)、miR-NC轉染組(1D)、未轉染組(1E)PANC1細胞的遷移(上)和侵襲能力(下)變化(×200)
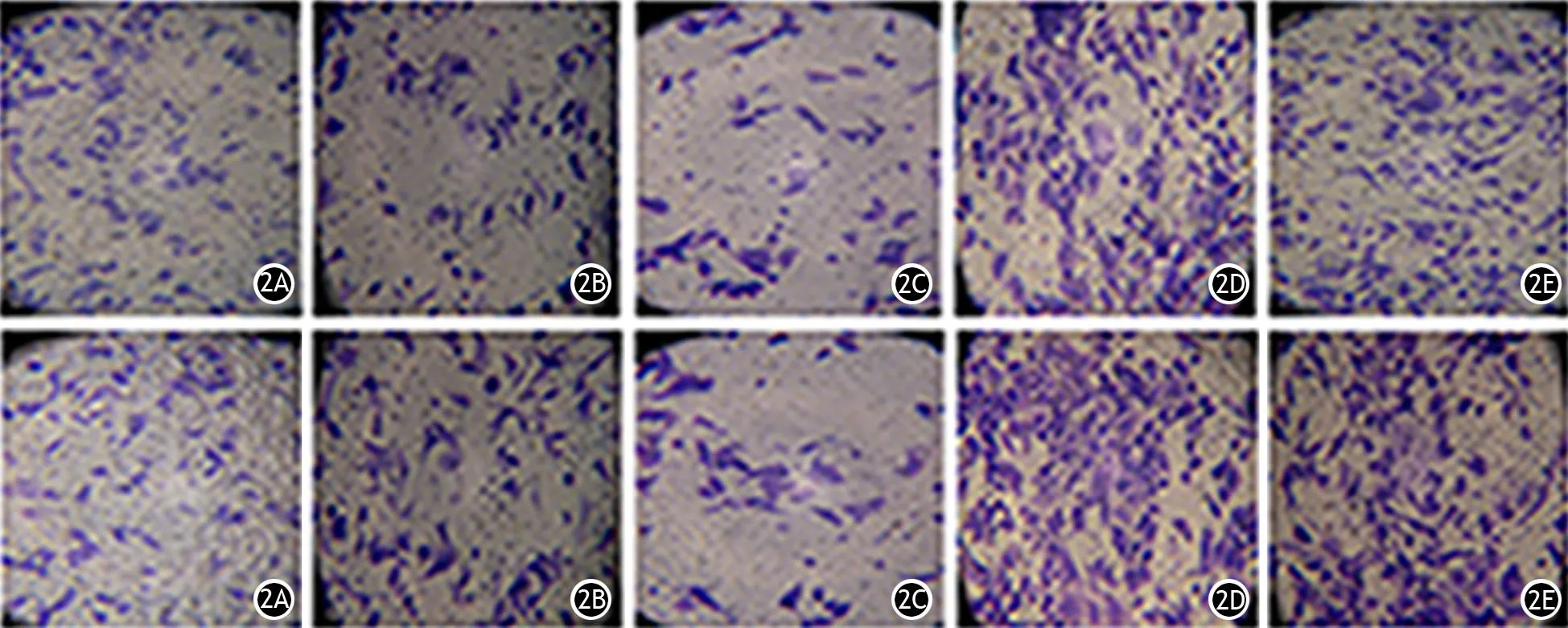
圖2 miR-34a轉染組(2A)、miR-let7轉染組(2B)、雙轉染組(2C)、miR-NC轉染組(2D)、未轉染組(2E)SW1990細胞的遷移(上)和侵襲能力(下)變化(×200)

細胞系miR-34a轉染組miR-let7轉染組雙轉染組miR-NC轉染組未轉染組PANC1103.70±3.28100.70±1.7661.67±3.18231.30±2.60153.70±2.60SW199094.67±3.18117.00±4.5051.00±2.08131.30±4.37160.70±4.63

表5 細胞遷移實驗中各轉染組穿過小室膜的細胞數(個/200倍視野,±s)
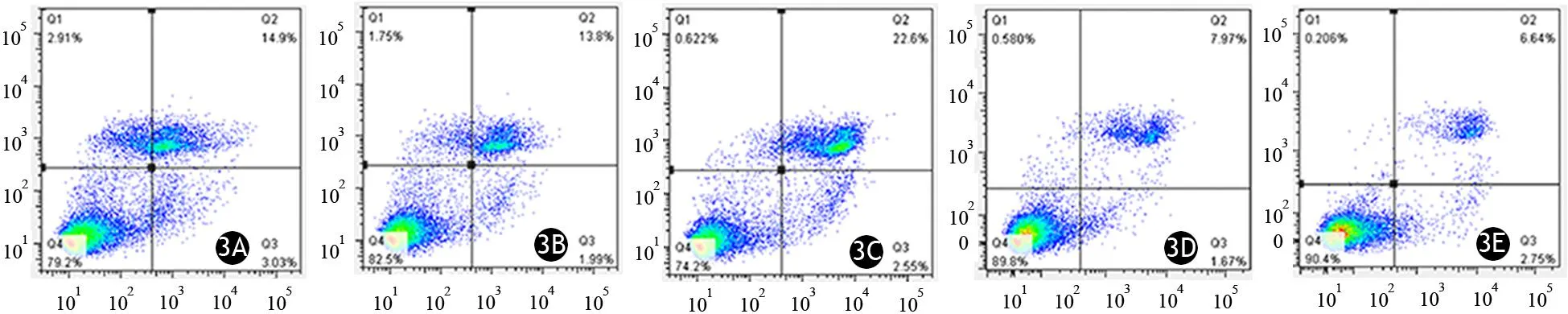
圖3 PANC1細胞的miR-34a轉染組(3A)、miR-let7轉染組(3B)、雙轉染組(3C)、miR-NC轉染組(3D)、未轉染組(3E)的細胞凋亡
討論
近年來隨著分子生物學領域的快速發展,胰腺癌的早診、早治已受到廣泛重視,針對胰腺癌分子水平的生物治療技術也取得了一定進展,為胰腺癌的治療提供了更新的理論基礎和實踐工具。盡管如此,多數新療法依然未得到廣泛認可,致使胰腺癌的早診、早治依然停留在臨床前研究階段,胰腺癌患者的生存率并無顯著改善[10]。究其原因,一方面在于腫瘤的發生、發展機制并不十分明確,另一方面在于多數分子靶向治療往往針對單一靶點,而未形成協同的抗腫瘤治療網絡,以致抗腫瘤效果不理想。
MicroRNA是一種內源性的小分子非編碼RNA,在轉錄后水平對細胞新陳代謝的多個環節進行調控。其表達譜的改變在胰腺癌細胞的增殖、凋亡、侵襲、分化等過程中發揮重要作用,與胰腺癌的發生、發展、預后等有著密切的聯系。有些miRNA,例如miR-34a、miR-203、miR-124、miR-let7在胰腺癌中表達降低,發揮抑癌基因作用[11-12],而另外一些miRNA,如miR-10a、miR-10b、miR-198、miR-21在胰腺癌組織中高表達,發揮癌基因的作用[13-14]。文獻報道,miR-34a在胰腺癌中表達缺失或明顯下調發揮著抑癌基因的作用,其靶基因CDK4、cyclins及E2F在調節細胞周期、細胞凋亡、DNA修復中有非常重要的作用[6,15-16]。Lize等[16]報道,miR-let7c及miR-let7f表達在人胰腺癌的穿刺標本中明顯降低,提高胰腺癌細胞株miR-let7的表達可顯著抑制癌細胞增殖[8]。miR-let7還與結腸癌、肝癌等有關[17],能夠抑制腫瘤細胞增殖,其作用可能與Ras、HMGA2和CRD-BP/IMP1的表達相關[18-20]。將miR-34a作用于胰腺癌細胞可抑制其皮下移植瘤的生長[21],利用質粒或慢病毒攜帶miRNA感染胰腺癌細胞增強其miR-let7的表達,能顯著抑制癌細胞的增殖[8]。Lou等[22]報道,通過溶瘤腺病毒共表達miR-34a及IL-24能夠發揮協同抗腫瘤活性,顯著提高二者抗腫瘤作用。因此,本研究同時使用兩種具有抑癌作用的miR-34a及miR-let7 轉染胰腺癌細胞,結果顯示雙轉染細胞增殖活性、細胞遷移和侵襲力均較單轉染細胞顯著下降,而細胞凋亡率較單轉染細胞顯著增加,能夠發揮更加顯著的協同抗腫瘤作用,為胰腺癌的治療提供新的思路。
參考文獻
[1]Yang L, Yang H, Li J, et al. ppENK gene methylation status in the development of pancreatic carcinoma[J]. Gastroenterol Res Pract, 2013, 2013: 130927.DOI:10.1155/2013/130927.
[2]Singh PK, Brand RE, Mehla K. MicroRNAs in pancreatic cancer metabolism[J]. Nat Rev Gastroenterol Hepatol, 2012, 9(6): 334-344.DOI:10.1038/nrgastro.2012.63.
[3]Zhang L, Jamaluddin MS, Weakley SM, et al. Roles and mechanisms of microRNAs in pancreatic cancer[J]. World J Surg, 2011, 35(8): 1725-1731.DOI:10.1007/s00268-010-0952-z.
[4]Du Y, Liu M, Gao J, et al. Aberrant microRNAs expression patterns in pancreatic cancer and their clinical translation[J]. Cancer Biother Radiopharm, 2013, 28(5): 361-369.
[5]Di Leva G, Croce CM. miRNA profiling of cancer[J]. Curr Opin Genet Dev, 2013, 23(1): 3-11.DOI:10.1016/j.gde.2013.01.004.
[6]Nana-Sinkam SP, Croce CM. Clinical applications for microRNAs in cancer[J]. Clin Pharmacol Ther, 2013, 93(1): 98-104.DOI:10.1038/clpt.2012.192.
[7]Tazawa H, Tsuchiya N, Izumiya M, et al. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells[J]. Proc Natl Acad U S A, 2007, 104(39): 15472-15477.DOI:10.1073/pnas.0707351104.
[8]Torrisani J, Bournet B, du Rieu MC, et al. let-7 MicroRNA transfer in pancreatic cancer-derived cells inhibits in vitro cell proliferation but fails to alter tumor progression[J]. Hum Gene Ther, 2009, 20(8): 831-844.DOI:10.1089/hum.2008.134.
[9]駱廣濤,王本忠. miRNA-4465過表達對乳腺癌MDA-MB-231細胞遷移侵襲的影響及其機制[J]. 安徽醫科大學學報, 2015, 50(11): 1570-1573.
[10]Michl P, Gress TM. Current concepts and novel targets in advanced pancreatic cancer[J]. Gut, 2013, 62(2): 317-326.DOI:10.1136/gutjnl-2012-303588.
[11]Xu D, Wang Q, An Y, et al. MiR203 regulates the proli-feration, apoptosis and cell cycle progression of pancreatic cancer cells by targeting Survivin[J]. Mol Med Rep, 2013, 8(2): 379-384.DOI:10.3892/mmr.2013.1504.
[12]Frampton AE, Krell J, Jamieson NB, et al. microRNAs with prognostic significance in pancreatic ductal adenocarcinoma: A meta-analysis[J]. Eur J Cancer, 2015, 51(11): 1389-1404.DOI:10.1016/j.ejca.2015.04.006.
[13]Ohuchida K, Mizumoto K, Lin C, et al. MicroRNA-10a is overexpressed in human pancreatic cancer and involved in its invasiveness partially via suppression of the HOXA1 gene[J]. Ann Surg Oncol, 2012, 19(7): 2394-2402.DOI:10.1245/s10434-012-2252-3.
[14]Vychytilova-Faltejskova P, Kiss I, Klusova S, et al. MiR-21, miR-34a, miR-198 and miR-217 as diagnostic and prognostic biomarkers for chronic pancreatitis and pancreatic ductal adenocarcinoma[J]. Diagn Pathol, 2015, 10: 38.DOI:10.1186/s13000-015-0272-6.
[15]Antonini D, Russo MT, De Rosa L, et al. Transcriptional repression of miR-34 family contributes to p63-mediated cell cycle progression in epidermal cells[J]. J Invest Dermatol, 2010, 130(5): 1249-1257.DOI:10.1038/jid.2009.438.
[16]Lize M, Pilarski S, Dobbelstein M. E2F1-inducible microRNA 449a/b suppresses cell proliferation and promotes apoptosis[J]. Cell Death Differ, 2010, 17(3): 452-458.DOI:10.1038/cdd.2009.188.
[17]Deng L, Yang SB, Xu FF, et al. Long noncoding RNA CCAT1 promotes hepatocellular carcinoma progression by functioning as let-7 sponge[J]. J Exp Clin Cancer Res, 2015, 34: 18.DOI:10.1186/s13046-015-0136-7.
[18]Boyerinas B, Park SM, Shomron N, et al. Identification of let-7-regulated oncofetal genes[J]. Cancer Res, 2008, 68(8): 2587-2591.DOI:10.1158/0008-5472.CAN-08-0264.
[19]Ma C, Nong K, Zhu H, et al. H19 promotes pancreatic cancer metastasis by derepressing let-7's suppression on its target HMGA2-mediated EMT[J]. Tumour Biol, 2014, 35(9): 9163-9169.DOI:10.1007/s13277-014-2185-5.
[20]Appari M, Babu KR, Kaczorowski A, et al. Sulforaphane, quercetin and catechins complement each other in elimination of advanced pancreatic cancer by miR-let-7 induction and K-ras inhibition[J]. Int J Oncol, 2014, 45(4): 1391-1400.DOI:10.3892/ijo.2014.2539.
[21]Hu QL, Jiang QY, Jin X, et al. Cationic microRNA-delivering nanovectors with bifunctional peptides for efficient treatment of PANC-1 xenograft model[J]. Biomaterials, 2013, 34(9): 2265-2276.DOI:10.1016/j.biomaterials.2012.12.016.
[22]Lou W, Chen Q, Ma L, et al. Oncolytic adenovirus co-expressing miRNA-34a and IL-24 induces superior antitumor activity in experimental tumor model[J]. J Mol Med, 2013, 91(6): 715-725.DOI:10.1007/s00109-012-0985-x.
(本文編輯:屠振興)
Effect of the combination of miR-34a and miR-let7 on the biological properties of pancreatic cancer cells
LiuYuting,ShenXiangguo,SuChangqing,SunBin,LiZhaoshen,XuCan.DepartmentofGastroenterology,ChanghaiHospital,SecondMilitaryMedicalUniversity,Shanghai200433China
【Abstract】ObjectiveTo investigate the influence on biological characteristics in human pancreatic cancer cells after beding transfected by two anti-carcinoma miRNAs at the same time. MethodsPancreatic cancer cells PANC1, SW1990 and normal pancreatic cells AR42J were transfected by miR-34a and(or) miR-let7 by liposome. Cells transfected with negative control miRNA (miR-NC) and untransfected were as controls. The expression of miR-34a and miR-let7 were detected by real-time fluorescent quantitative RT-PCR. The cell proliferation was detected by MTT test and the migration and invasion were evaluated by transwell assay. The apoptosis rate was measured by flow cytometric analysis. ResultsAfter being transfected with miRNAs, the expression of miR-34a and miR-let7 in double transfection group (miR-34a and miR-let7 were transfected at the same time). miR-34a transfection group, miR-let7 transfection group was significantly up-regulated than those in miRNA-NC transfection group and untransfected group in PANC1 cells, SW1990 cells and AR42J cells, repectively. The difference which was statistically significant(P<0.05)indicating that cells were successfully transfected. The cell proliferation in double transfection group of PANC1 cells and SW1990 cells were (0.665±0.01, 0.6375±0.03), which were significantly inhibited compared with (0.974±0.03, 0.971±0.05) in miR-NC group and (0.8875±0.05, 0.8625±0.06) in miR-let7 group. The difference was statistically significant(P<0.05). The cell proliferation activity in double transfection group was lower than those in miR-34a group (0.795±0.06, 0.7925±0.06), but did not have statistically significant difference. There was no significant change in AR42J cells. Cell invasion assay showed that the number of PANC1 cells permeating substrate membrane in miR34a group (103.7±3.28) and miR-let7 group (100.7±1.76) were significantly fewer than miR-NC group (231.3±2.6) and untransfected group (153.7±2.6). The number of cells permeating substrate membrane in double transfection group(61.67±3.18)was fewer than miR-34a group and miR-let7 group, respectively. The difference was statistically significant(P<0.01).The migration test had consistent results with invasion test. The changes of invasion and migration in SW1990 cells were similar to those in PANC1 cells. The apoptosis rate of PANC1 cells in miR-34a group, miR-let7 group, double transfection group, miR-NC group and untransfected group was (16.66±1.27)%,(15.46±0.33)%,(23.35±1.80)%,(9.33±0.31)% and (8.83±0.36)% respectively. Single transfection group had higher apoptosis rate than miR-NC group and untransfected group (P<0.05). Double transfection group had a significantly higher apoptosis rate than miR-let7 group (P<0.05), while there was no significant difference between double transfection group and miR-34a group. ConclusionsThe cell proliferation, invasion and migration in double miRNAs transfected pancreatic cancer cells were significantly down-regulated compared with those in single miRNA transfected cells, while apoptosis rate in double miRNAs transfection group was higher than single miRNA transfection group. Thus, the combination of two anti-cancer miRNAs may exert a more significant synergistic antitumor effect.
【Key words】Pancreatic neoplasms;Cell line, tumor;miR-34a;miR-let7;Transfection;Molecular biology
(收稿日期:2015-12-15)
Corresponding author:Xu Can, Email: xxcc211@126.com
基金項目:國家自然科學基金面上項目(81372673);上海市浦江人才計劃項目(14PJD004)
通信作者:徐燦,Email: xxcc211@126.com
DOI:10.3760/cma.j.issn.1674-1935.2016.02.004
Fund program:National Natural Sceince Foundation of China(81372673);Shanghai Pujiang Talent Program Item(14PJD004)

