MiR-126通過MAPK信號通路抑制oxLDL處理的血管平滑肌細(xì)胞增殖和遷移
鄭 茜 張 勇 楊東偉
(鄭州大學(xué)附屬鄭州中心醫(yī)院心血管內(nèi)科,鄭州 450000)
越來越多的研究表明小RNA(microRNAs,miRNAs)在動脈粥樣硬化的病理生理細(xì)胞效應(yīng)及分子信號通路中起著重要的調(diào)控作用[1]。MiRNAs是一類小非編碼RNA序列,長約22個核苷酸,目前已發(fā)現(xiàn)2 042種miRNAs[2]。這些miRNAs共同調(diào)控著基因組中三分之一的基因,而且miRNAs與多種疾病相關(guān)[3]。MiRNAs在神經(jīng)性疾病、哮喘及癌癥等疾病中都起著重要作用[4-8]。MiR-126在多種癌癥中都低表達(dá),可通過抑制癌細(xì)胞生長、遷移及侵襲等影響癌癥進(jìn)程[9]。MiR-126在心血管疾病中也具有重要作用。有研究發(fā)現(xiàn)血漿中的miR-126水平與冠狀動脈側(cè)支循環(huán)形成呈正相關(guān)[10]。有數(shù)據(jù)顯示在伴有多種血管疾病的冠狀動脈疾病患者中miR-126低表達(dá)[11]。在心肌缺血再灌注損傷中,miR-126表達(dá)下調(diào),miR-126可保護(hù)由缺血再灌注引發(fā)的心肌細(xì)胞凋亡[12]。大量文獻(xiàn)報道m(xù)iR-126具有保護(hù)血管和動脈粥樣硬化的功能[13]。過表達(dá)miR-126可減弱靜脈內(nèi)皮細(xì)胞凋亡抑制深靜脈血栓形成[14]。有研究表明內(nèi)皮微顆粒可通過向受體細(xì)胞輸送功能性的miR-126來促進(jìn)血管內(nèi)皮修復(fù)[15]。本文主要目的是利用氧化型低密度脂蛋白(oxidized low density lipoprotein,oxLDL)處理人主動脈血管平滑肌細(xì)胞(Vascular smooth muscle cell,VSMC),研究miR-126對oxLDL處理的VSMC生物學(xué)功能的影響及其分子機制。
1 材料與方法
1.1細(xì)胞系及主要試劑 人主動脈血管平滑肌細(xì)胞系T/G HAVSMC購自美國典型培養(yǎng)物保藏中心(American Type Culture Collection,ATCC);oxLDL購自美國Kalen Biomedical公司;促分裂原活化蛋白激酶(Mitogen-activated protein kinase,MAPK)信號通路激活劑Anisomycin購自碧云天生物科技公司;SmGM-2培養(yǎng)基購自瑞士Lonza龍沙公司;胎牛血清及轉(zhuǎn)染試劑TurboFect Transfection Regent購自賽默飛世爾科技公司;血脂檢測試劑盒購自南京建成生物工程研究所;RNA提取試劑盒RNAiso Plus reagent購自大連TaKaRa公司;CCK-8試劑盒購自日本同仁化學(xué)公司;Transwell小室購自美國BD公司;增殖標(biāo)記蛋白細(xì)胞增殖核抗原-67(Antigen identified by monoclonal antibody,Ki-67)和增殖細(xì)胞核抗原(Proliferating cell nuclear antigen,PCNA),遷移標(biāo)記蛋白基質(zhì)金屬蛋白酶9(Matrix metalloprotein 9,MMP-9)和血管內(nèi)皮細(xì)胞生長因子(Vascular endothelial growth factor,VEGF)抗體購自美國NEB公司;MAPK信號通路相關(guān)蛋白ERK1/2、p-ERK1/2、p38、p-p38、JNK和p-JNK抗體購自英國Abcam公司。
1.2方法
1.2.1細(xì)胞培養(yǎng)及處理和轉(zhuǎn)染 人主動脈血管平滑肌細(xì)胞T/G HAVSMC于含5%胎牛血清的SmGM-2培養(yǎng)基中置于37℃、5%CO2的恒溫培養(yǎng)箱中培養(yǎng)。用生理鹽水處理T/G HAVSMC細(xì)胞24 h作為對照組,用50 mg/ml的oxLDL處理T/G HAVSMC細(xì)胞24 h作為模型組。依照轉(zhuǎn)染試劑TurboFect Transfection Regent說明書用miR-126 mimic和mimic control分別對模型組細(xì)胞進(jìn)行轉(zhuǎn)染并添加MAPK信號通路激活劑Anisomycin。
1.2.2膽固醇及三酰甘油水平檢測 oxLDL處理T/G HAVSMC后收集細(xì)胞上清,按照試劑盒說明書測定總膽固醇(Serum total cholesterol,TC)、低密度脂蛋白膽固醇(Low density lipoprotein cholesterol,LDL-C)、高密度脂蛋白膽固醇(High-density lipopro-tein cholesterol,HDL-C)和三酰甘油(Triglyceride,TG)。
1.2.3實時定量PCR(Quantitative real-time reverse transcription PCR,qRT-PCR) 用RNA提取試劑盒提取總RNA并反轉(zhuǎn)成cDNA,以cDNA為模板進(jìn)行qRT-PCR。miR-126上游引物:GCTGTCAGTTTGTC-AAATAC,miR-126下游引物:GTGCAGGGTCC-GAGGT。根據(jù)SYBR Premix Ex TaqTM說明書進(jìn)行qRT-PCR,用公式2-ΔΔCt計算miR-126的相對表達(dá)量。
1.2.4CCK-8檢測細(xì)胞增殖 轉(zhuǎn)染0、1、2、3、4、5 d后,CCK-8檢測各組細(xì)胞增殖倍數(shù)。首先將CCK-8溶液稀釋到10%,然后用上述稀釋液將待測的細(xì)胞制成1×106個/ml的懸液,37℃培養(yǎng)1~4 h,最后450 nm處檢測吸光值,計算細(xì)胞增殖倍數(shù)。
1.2.5Transwell分析細(xì)胞遷移 待測細(xì)胞懸浮于無胎牛血清的培養(yǎng)基中至細(xì)胞密度為1×106個/ml,然后將細(xì)胞懸液加入到Transwell的上室中,在下室中加入含10%胎牛血清的培養(yǎng)基。37℃培養(yǎng)24 h后,0.5%的結(jié)晶紫對上室底部細(xì)胞進(jìn)行染色,并用棉簽將上室內(nèi)側(cè)的細(xì)胞除去。顯微鏡下觀察細(xì)胞形態(tài)并隨機選取5個視野統(tǒng)計每個視野下被染色的細(xì)胞數(shù)量。
1.2.6免疫印記 收集待測細(xì)胞,用PBS洗3次,然后加入已添加蛋白酶抑制劑的細(xì)胞裂解液進(jìn)行裂解后提取總蛋白。等量的蛋白進(jìn)行SDS-PAGE凝膠電泳分離,然后轉(zhuǎn)至PVDF膜。用5%的BSA進(jìn)行封閉后,依次孵育一抗和二抗,最后進(jìn)行顯色。
1.3統(tǒng)計學(xué)分析 用SPSS16.0軟件對實驗數(shù)據(jù)進(jìn)行統(tǒng)計學(xué)分析,兩兩比較用獨立的t檢驗。P<0.05表示差異有統(tǒng)計學(xué)意義。
2 結(jié)果
2.1oxLDL對VSMC脂質(zhì)代謝的影響 用50 mg/ml的oxLDL處理T/G HAVSMC細(xì)胞24 h后檢測模型組和對照組中TC、TG、LDL-C、HDL-C的濃度。如表1所示,模型組中TC、TG和LDL-C的濃度遠(yuǎn)高于對照組,HDL-C的濃度則現(xiàn)顯著低于對照組(P<0.001)。這些指標(biāo)的變化說明,oxLDL處理T/G HAVSMC后脂質(zhì)代謝異常。
2.2oxLDL處理VSMC可降低miR-126表達(dá) 利用qRT-PCR檢測對照組和模型組中miR-126的表達(dá),如圖1所示,模型組中miR-126相對表達(dá)量顯著降低(P<0.01)。由此可見,在oxLDL處理T/G HAVSMC的模型中miR-126下調(diào)表達(dá)。
2.3增強oxLDL處理的VSMC中miR-126的表達(dá) 將miR-126 mimic和mimic control分別轉(zhuǎn)染模型組細(xì)胞,qRT-PCR檢測各組細(xì)胞miR-126的表達(dá)情況。如圖2所示,模型組和mimic control組中miR-126的相對表達(dá)量顯著低于對照組(P<0.01)。miR-126 mimic組中miR-126的相對表達(dá)量遠(yuǎn)遠(yuǎn)高于模型組(P<0.001)。這些結(jié)果說明,miR-126 mimic轉(zhuǎn)染效果顯著,大大地提高了模型組細(xì)胞miR-126的表達(dá)。

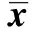

GroupsTC(mmol/L)TG(mmol/L)LDL?C(mmol/L)HDL?C(mmol/L)Control1 28±0 080 84±0 090 73±0 110 61±0 07Model6 78±0 161)5 89±0 181)7 08±0 231)0 39±0 051)
Note:TC.Total cholesterol;TG.Triglyceride;LDL-C.Low density lipoprotein cholesterin;HDL-C.High density lipoprotein cholesterol.Compared with control group,1)P<0.001.
2.4miR-126對oxLDL誘導(dǎo)的VSMC增殖的影響 為分析miR-126在oxLDL處理的VSMC中對細(xì)胞增殖的影響,利用CCK-8檢測各組細(xì)胞增殖倍數(shù)。圖3結(jié)果顯示,與對照組相比,oxLDL誘導(dǎo)的動脈粥樣硬化模型組和mimic control組細(xì)胞增殖倍數(shù)在轉(zhuǎn)染后第2天開始明顯增加(P<0.05),轉(zhuǎn)染后第3天則顯著增多(P<0.01),轉(zhuǎn)染4 d以后則極顯著升高了(P<0.001)。模型組中轉(zhuǎn)染miR-126 mimic后第3天開始細(xì)胞增殖倍數(shù)明顯低于模型組(P<0.05),轉(zhuǎn)染4 d以后增殖倍數(shù)顯著減少(P<0.01)。由此可見,miR-126 mimic可抑制oxLDL誘導(dǎo)的VSMC增殖的增加。

圖1 qRT-PCR檢測oxLDL處理后miR-126的表達(dá)Fig.1 Expression of miR-126 was tested by qRT-PCR after treatment with oxLDLNote: Compared with control group,**.P<0.01.

圖2 qRT-PCR檢測轉(zhuǎn)染miR-126 mimic后miR-126的表達(dá)Fig.2 Expression of miR-126 was tested by qRT-PCR after transfection with miR-126 mimicNote: Compared with control group,**.P<0.01;comprared with model group,###.P<0.001.
2.5miR-126在oxLDL處理的VSMC中對細(xì)胞遷移的影響 Transwell檢測各組細(xì)胞遷移結(jié)果顯示,模型組和mimic control組遷移細(xì)胞數(shù)最多,其次是miR-126 mimic組,對照組遷移細(xì)胞數(shù)最少(圖4A)。如圖4B所示,模型組和mimic control組中平均每個視野的遷移細(xì)胞數(shù)遠(yuǎn)遠(yuǎn)高于對照組(P<0.001)。與模型組相比,miR-126 mimic組遷移細(xì)胞數(shù)顯著降低(P<0.01)。上述結(jié)果表明,miR-126 mimic可減弱oxLDL對VSMC遷移能力的促進(jìn)作用。
2.6miR-126對細(xì)胞增殖和遷移標(biāo)記蛋白表達(dá)的影響 為進(jìn)一步驗證miR-126在oxLDL處理的VSMC中對細(xì)胞增殖和遷移的影響,免疫印跡檢測各組細(xì)胞增殖和遷移標(biāo)記蛋白表達(dá)。圖5A顯示,增殖和遷移標(biāo)記蛋白Ki-67、PCNA、MMP-9、VEGF在模型組和mimic control組中表達(dá)最強,miR-126 mimic組較低,對照組表達(dá)最弱。由圖5B可知,模型組和mimic control組中Ki-67、PCNA、MMP-9、VEGF的相對表達(dá)量顯著高于對照組(P<0.01)。與模型組相比,miR-126 mimic組Ki-67、PCNA、MMP-9、VEGF的相對表達(dá)量明顯減少(P<0.05)。這些結(jié)果說明,oxLDL可增強VSMC中細(xì)胞增殖和遷移標(biāo)記蛋白表達(dá)。

圖3 CCK-8檢測細(xì)胞增殖Fig.3 Cell proliferation was detected by CCK-8Note: Compared with control group,*.P<0.05,**.P<0.01,***.P<0.001;compared with model group,#.P<0.05,##.P<0.01.


圖4Transwell檢測細(xì)胞遷移(結(jié)晶紫染色,×100)
Fig.4MigrationwasmeasuredbyTranswell(crystalvioletstaining,×100)
Note: A.Crystal violet staining;B.Histogram represents the statistical analysis of Transwell migration.Compared with model group,***.P<0.001;compared with model group,##.P<0.01.
2.7miR-126對MAPK信號通路相關(guān)蛋白表達(dá)的影響 為探索miR-126在oxLDL處理的VSMC中的分子調(diào)控機制,免疫印跡分析MAPK信號通路相關(guān)蛋白的表達(dá)。如圖6A所示,磷酸化的蛋白p-ERK1/2、p-p38、p-JNK在模型組和mimic control組表達(dá)量最多,其次是miR-126 mimic,對照組中表達(dá)量最少,但各組細(xì)胞中ERK1/2、p38、JNK蛋白表達(dá)無明顯變化。圖6B顯示,與對照組相比,模型組和mimic control組中p-ERK1/2/ERK1/2、p-p38/p38、p-JNK/JNK的相對蛋白表達(dá)量的比值顯著升高(P<0.01),miR-126 mimic組p-ERK1/2/ERK1/2、p-p38/p38、p-JNK/JNK的相對蛋白表達(dá)量的比值則明顯低于模型組(P<0.05)。由此可見,miR-126 mimic可降低oxLDL引起的VSMC中MAPK信號通路相關(guān)蛋白磷酸化水平的升高,抑制MAPK信號通路活化。

圖5 免疫印跡檢測細(xì)胞增殖和遷移標(biāo)記蛋白的表達(dá)Fig.5 Expression of proliferation and migration marker proteins was tested by Western blotNote: A.Representative graph of Western blot;B.Histogram represents the statistical analysis of Western blot.Compared with control group,**.P<0.01;compared with model group,#.P<0.05.

圖6 免疫印跡檢測MAPK信號通路相關(guān)蛋白的表達(dá)Fig.6 Expression of MAPK pathway related proteins was tested by Western blotNote: A.Representative graph of Western blot;B.Histogram represents the statistical analysis of Western blot.Compared with control group,**;P<0.01;compared with model group,#.P<0.05.

圖7 免疫印跡檢測細(xì)胞增殖和遷移標(biāo)記蛋白的表達(dá)Fig.7 Expression of proliferation and migration marker proteins was tested by Western blotNote: A.Representative graph of Western blot;B.Histogram represents the statistical analysis of Ki-67 and PCNA;C.Histogram represents the statistical analysis of MMP-9 and VEGF,compared with control group,**.P<0.01,compared with model group,#.P<0.05.
2.8miR-126通過MAPK信號通路發(fā)揮作用 為進(jìn)一步確認(rèn)miR-126是通過MAPK信號通路來發(fā)揮作用,添加MAPK信號通路激活劑Anisomycin檢測各組細(xì)胞增殖和遷移標(biāo)記蛋白表達(dá)。圖7A顯示,增殖和遷移標(biāo)記蛋白Ki-67、PCNA、MMP-9、VEGF在Anisomycin組中表達(dá)最高。如圖7B和7C所示,與對照組相比,模型組Ki-67、PCNA、MMP-9、VEGF表達(dá)明顯升高(P<0.05)。與模型組相比,miR-126 mimic組Ki-67、PCNA、MMP-9、VEGF表達(dá)明顯降低(P<0.05);Anisomycin組Ki-67、PCNA、MMP-9、VEGF表達(dá)明顯升高(P<0.05)。miR-mimic+ Anisomycin組Ki-67、PCNA、MMP-9、VEGF表達(dá)與模型組無明顯差異。由此可見,MAPK信號通路激活劑Anisomycin可逆轉(zhuǎn)miR-126 mimic對oxLDL處理的VSMC增殖和遷移的影響。上述結(jié)果表明,miR-126可通過抑制MAPK信號通路減弱oxLDL誘導(dǎo)的VSMC增殖和遷移。
3 討論
動脈粥樣硬化的發(fā)生發(fā)展過程中會出現(xiàn)內(nèi)皮細(xì)胞功能紊亂,平滑肌細(xì)胞增殖和遷移,炎性細(xì)胞招募,脂質(zhì)和基質(zhì)的積累以及血栓的形成[16]。針對這些變化,目前對于動脈粥樣硬化的治療策略主要包括生活方式的改變、降膽固醇降血壓藥物和抗血栓藥物以及外科手術(shù)治療,但是這些方法并非對所有的患者有效[17]。尋找開發(fā)新的治療方案具有重大意義。隨著分子生物學(xué)水平的提高,基因治療已經(jīng)成為一種極具潛力的疾病治療手段。表達(dá)譜分析表明,在動脈粥樣硬化的病理背景下,許多miRNAs在內(nèi)皮細(xì)胞、平滑肌細(xì)胞和巨噬細(xì)胞中都表達(dá)異常[18]。這揭示這些miRNAs可能在動脈粥樣硬化中起著重要作用,具有成為治療靶點的可能。
大量文獻(xiàn)報道了miRNAs在多種疾病中對細(xì)胞增殖具有重要的調(diào)控作用。在胰島瘤細(xì)胞中,miR-126可抑制葡萄糖引起的細(xì)胞增殖[19]。Yu等[20]發(fā)現(xiàn)miR-126上調(diào)表達(dá)會導(dǎo)致宮頸癌細(xì)胞增殖能力降低。miR-126還可調(diào)節(jié)類風(fēng)濕性關(guān)節(jié)炎骨膜纖維母細(xì)胞增殖[21]。Schober等[22]發(fā)現(xiàn)miR-126可促進(jìn)內(nèi)皮增生并抑制動脈粥樣硬化病變。血管平滑肌細(xì)胞的增殖是動脈粥樣硬化的關(guān)鍵過程之一[23]。有數(shù)據(jù)表明oxLDL處理血管平滑肌細(xì)胞會導(dǎo)致miR-141表達(dá)降低,miR-141對血管平滑肌細(xì)胞增殖起著重要的調(diào)控作用[24]。Kim等[25]研究顯示過表達(dá)miR-365可降低血管平滑肌細(xì)胞增殖能力和增殖標(biāo)記蛋白PCNA的表達(dá)。過表達(dá)miR-599可抑制血管平滑肌細(xì)胞增殖及增殖標(biāo)記蛋白PCNA和Ki-67的表達(dá)[26]。與前人結(jié)果類似,本文結(jié)果顯示,50 mg/L的oxLDL處理人主動脈血管平滑肌細(xì)胞24 h會導(dǎo)致miR-126表達(dá)減弱,細(xì)胞增殖倍數(shù)及增殖標(biāo)記蛋白表達(dá)升高;而miR-126 mimic則可以降低oxLDL誘導(dǎo)的細(xì)胞增殖倍數(shù)和增殖標(biāo)記蛋白表達(dá)的增加。這說明,miR-126 mimic在可抑制oxLDL誘導(dǎo)的人主動脈血管平滑肌細(xì)胞T/G HAVSMC增殖能力的升高。
MiRNAs對多種疾病的細(xì)胞遷移也存在很大的影響。在結(jié)腸直腸癌中,增強miR-126表達(dá)可以抑制結(jié)腸直腸癌細(xì)胞遷移能力[27]。過表達(dá)miR-126還可抑制骨肉瘤細(xì)胞遷移[28]。Yang等[29]發(fā)現(xiàn)miR-126可抑制胃癌細(xì)胞遷移能力。血管平滑肌細(xì)胞是動脈管壁最豐富的細(xì)胞且與動脈粥樣硬化有關(guān)。據(jù)報道m(xù)iRNAs還可調(diào)控動脈粥樣硬化中平滑肌細(xì)胞的遷移[30]。有研究顯示miR-379可以抑制血管平滑肌細(xì)胞遷移[31]。過表達(dá)miR-145和miR-181b會導(dǎo)致血管平滑肌細(xì)胞遷移能力降低[32,33]。Cho等[34]發(fā)現(xiàn)miR-761可抑制血管緊縮素引起的血管平滑肌細(xì)胞遷移能力的增強。本研究結(jié)果顯示,oxLDL處理人主動脈血管平滑肌細(xì)胞后,細(xì)胞遷移及遷移標(biāo)記蛋白表達(dá)增強;miR-126 mimic則可以抑制這種oxLDL誘導(dǎo)的細(xì)胞遷移及遷移標(biāo)記蛋白表達(dá)的升高。這表明,增強miR-126表達(dá)可減弱oxLDL導(dǎo)致的人主動脈血管平滑肌細(xì)胞T/G HAVSMC遷移能力的升高。
眾所周知MAPK信號通路具有重要的生物學(xué)功能。據(jù)報道許多miRNAs是通過MAPK信號通路來發(fā)揮其功能。有研究表明過表達(dá)miR-126可通過調(diào)節(jié)ERK MAPK信號通路促進(jìn)間質(zhì)干細(xì)胞向內(nèi)皮細(xì)胞分化[35]。Zuo等[36]發(fā)現(xiàn)在動脈粥樣硬化中,過表達(dá)內(nèi)皮祖細(xì)胞的miR-26a會導(dǎo)致p-p38水平下降,p38表達(dá)水平無變化,這說明miR-26a可通過p38 MAPK途徑調(diào)控內(nèi)皮祖細(xì)胞功能。有數(shù)據(jù)顯示在動脈粥樣硬化中miR-136可已通過ERK1/2 MAPK通路調(diào)節(jié)血管平滑肌細(xì)胞增殖[37]。Li等[38]發(fā)現(xiàn)miR-181b可通過調(diào)節(jié)p-ERK1/2/ERK1/2平衡來調(diào)控血管平滑肌細(xì)胞增殖。本文結(jié)果顯示,在oxLDL處理的VSMC中,MAPK信號通路中的p-ERK1/2/ERK1/2、p-p38/p38、p-JNK/JNK的相對蛋白表達(dá)量的比值升高,轉(zhuǎn)染miR-126 mimic可降低MAPK信號通路相關(guān)蛋白磷酸化水平的增加。而且,MAPK信號通路激活劑Anisomycin可逆轉(zhuǎn)miR-126 mimic對oxLDL處理的VSMC增殖和遷移的影響。上述結(jié)果表明,miR-126可通過MAPK信號通路調(diào)節(jié)人主動脈血管平滑肌細(xì)胞的增殖和遷移。
本研究表明,oxLDL處理人主動脈血管平滑肌細(xì)胞的模型中,細(xì)胞增殖和遷移能力提高,MAPK信號通路中的p-ERK1/2/ERK1/2、p-p38/p38、p-JNK/JNK比值升高。MiR-126 mimic可降低動脈粥樣硬化中細(xì)胞增殖和遷移能力及MAPK信號通路相關(guān)蛋白磷酸化水平的升高。MAPK信號通路激活劑Anisomycin可逆轉(zhuǎn)miR-126 mimic對oxLDL處理的VSMC增殖和遷移的影響。
綜上所述,miR-126可通過MAPK信號通路抑制oxLDL誘導(dǎo)的VSMC增殖和遷移。下一步計劃將通過動物模型對miR-126在動脈粥樣硬化中的作用進(jìn)行體內(nèi)研究,為開發(fā)新的動脈粥樣硬化治療方案奠定基礎(chǔ)。
[1] Feinberg MW,Moore KJ.MicroRNA regulation of atherosclerosis[J].Circ Res,2016,118(4):703-720.
[2] Di Leva G,Garofalo M,Croce CM.MicroRNAs in cancer[J].Annu Rev Pathol,2014,9:287-314.
[3] Hammond SM.An overview of microRNAs[J].Adv Drug Deliv Rev,2015,87:3-14.
[4] Greenberg DS,Soreq H.MicroRNA therapeutics in neurological disease[J].Curr Pharm Des,2014,20(38):6022-6027.
[5] Igaz P,Igaz I,Nagy Z,etal.MicroRNAs in adrenal tumors:relevance for pathogenesis,diagnosis,and therapy[J].Cell Mol Life Sci,2015,72(3):417-428.
[6] Kai W,Qian XU,Qun WU.MicroRNAs and asthma regulation[J].Iran J Allergy Asthma Immunol,2015,14(2):120-125.
[7] Perge P,Nagy Z,Igaz I,etal.Suggested roles for microRNA in tumors[J].Biomol Concepts,2015,6(2):149-155.
[8] Sellitti DF,Doi SQ.MicroRNAs in renal cell carcinoma[J].Microrna,2015,4(1):26-35.
[9] Ebrahimi F,Gopalan V,Smith RA,etal.miR-126 in human cancers:clinical roles and current perspectives[J].Exp Mol Pathol,2014,96(1):98-107.
[10] Nie X,Su L,Zhou Y,etal.Association between plasma levels of microRNA-126 and coronary collaterals in patients with coronary artery disease[J].Zhonghua Xin Xue Guan Bing Za Zhi,2014,42(7):561-565.
[11] Li HY,Zhao X,Liu YZ,etal.Plasma MicroRNA-126-5p is associated with the complexity and severity of coronary artery disease in patients with stable angina pectoris[J].Cell Physiol Biochem,2016,39(3):837-846.
[12] Li B,Tao Y,Huang Q.Effect and mechanism of miR-126 in myocardial ischemia reperfusion[J].Genet Mol Res,2015,14(4):18990-18998.
[13] Chistiakov DA,Orekhov AN,Bobryshev YV.The role of miR-126 in embryonic angiogenesis,adult vascular homeostasis,and vascular repair and its alterations in atherosclerotic disease[J].J Mol Cell Cardiol,2016,97:47-55.
[14] Chen L,Wang J,Wang B,etal.MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling[J].Ann Hematol,2016,95(3):365-374.
[15] Jansen F,Yang X,Hoelscher M,etal.Endothelial microparticle-mediated transfer of MicroRNA-126 promotes vascular endothelial cell repair via SPRED1 and is abrogated in glucose-damaged endothelial microparticles[J].Circulation,2013,128(18):2026-2038.
[16] Mani S,Untereiner A,Wu L,etal.Hydrogen sulfide and the pathogenesis of atherosclerosis[J].Antioxid Redox Signal,2014,20(5):805-817.
[17] Kivela AM,Huusko J,Yla-Herttuala S.Prospect and progress of gene therapy in treating atherosclerosis[J].Exp Opin Biol Ther,2015,15(12):1699-1712.
[18] Loyer X,Mallat Z,Boulanger CM,etal.MicroRNAs as therapeutic targets in atherosclerosis[J].Exp Opin Ther Targets,2015,19(4):489-496.
[19] Tao H,Wang MM,Zhang M,etal.MiR-126 suppresses the glucose-stimulated proliferation via IRS-2 in INS-1 beta cells[J].PLoS One,2016,11(2):e0149954.
[20] Yu Q,Liu SL,Wang H,etal.miR-126 suppresses the proliferation of cervical cancer cells and alters cell sensitivity to the chemotherapeutic drug bleomycin[J].Asian Pac J Cancer Prev,2014,14(11):6569-6572.
[21] Qu Y,Wu J,Deng JX,etal.MicroRNA-126 affects rheumatoid arthritis synovial fibroblast proliferation and apoptosis by targeting PIK3R2 and regulating PI3K-AKT signal pathway[J].Oncotarget,2016,7(45):74217-74226.
[22] Schober A,Nazari-Jahantigh M,Wei Y,etal.MicroRNA-126-5p promotes endothelial proliferation and limits atherosclerosis by suppressing Dlk1[J].Nat Med,2014,20(4):368-376.
[23] Xu Z,Han Y,Liu J,etal.MiR-135b-5p and MiR-499a-3p promote cell proliferation and migration in atherosclerosis by directly targeting MEF2C[J].Sci Rep,2015,5:12276.
[24] Zhang Y,Chen B,Ming L,etal.MicroRNA-141 inhibits vascular smooth muscle cell proliferation through targeting PAPP-A[J].Int J Clin Exp Pathol,2015,8(11):14401-14408.
[25] Kim MH,Ham O,Lee SY,etal.MicroRNA-365 inhibits the proliferation of vascular smooth muscle cells by targeting cyclin D1[J].J Cell Biochem,2014,115(10):1752-1761.
[26] Xie B,Zhang C,Kang K,etal.miR-599 inhibits vascular smooth muscle cells proliferation and migration by targeting TGFB2[J].PLoS One,2015,10(11):e0141512.
[27] Li Z,Li N,Wu M,etal.Expression of miR-126 suppresses migration and invasion of colon cancer cells by targeting CXCR4[J].Mol Cell Biochem,2013,381(1-2):233-242.
[28] Jiang R,Zhang C,Liu G,etal.MicroRNA-126 inhibits proliferation,migration,invasion,and EMT in osteosarcoma by targeting ZEB1[J].J Cell Biochem,2017,118(11):3765-3774.
[29] Yang Z,Wang R,Zhang T,etal.MicroRNA-126 regulates migration and invasion of gastric cancer by targeting CADM1[J].Int J Clin Exp Pathol,2015,8(8):8869-8880.
[30] Yu X,Li Z.MicroRNAs regulate vascular smooth muscle cell functions in atherosclerosis(review)[J].Int J Mol Med,2014,34(4):923-933.
[31] Li K,Wang Y,Zhang A,etal.miR-379 inhibits cell proliferation,invasion,and migration of vascular smooth muscle cells by targeting insulin-like factor-1[J].Yonsei Med J,2017,58(1):234-240.
[32] Li Y,Huang J,Jiang Z,etal.MicroRNA-145 regulates platelet-derived growth factor-induced human aortic vascular smooth muscle cell proliferation and migration by targeting CD40[J].Am J Transl Res,2016,8(4):1813-1825.
[33] Li X,Cao G.Potential role of microRNA-181b on atherosclerosis[J].Zhonghua Xin Xue Guan Bing Za Zhi,2015,43(6):516-520.
[34] Cho JR,Lee CY,Lee J,etal.MicroRNA-761 inhibits Angiotensin II-induced vascular smooth muscle cell proliferation and migration by targeting mammalian target of rapamycin[J].Clin Hemorheol Microcirc,2015,63(1):45-56.
[35] Huang F,F(xiàn)ang ZF,Hu XQ,etal.Overexpression of miR-126 promotes the differentiation of mesenchymal stem cells toward endothelial cells via activation of PI3K/Akt and MAPK/ERK pathways and release of paracrine factors[J].Biol Chem,2013,394(9):1223-1233.
[36] Zuo K,Zhi K,Zhang X,etal.A dysregulated microRNA-26a/EphA2 axis impairs endothelial progenitor cell function via the p38 MAPK/VEGF pathway[J].Cell Physiol Biochem,2015,35(2):477-488.
[37] Zhang CF,Kang K,Li XM,etal.MicroRNA-136 promotes vascular muscle cell proliferation through the ERK1/2 pathway by targeting PPP2R2A in atherosclerosis[J].Curr Vasc Pharmacol,2015,13(3):405-412.
[38] Li TJ,Chen YL,Gua CJ,etal.MicroRNA 181b promotes vascular smooth muscle cells proliferation through activation of PI3K and MAPK pathways[J].Int J Clin Exp Pathol,2015,8(9):10375-10384.

