Effect of Exopolysaccharide Produced by Lactobacillus casei HS4 on Microstructure and Rheological Properties of Fermented Milk
BAI Ying, LIU Naiqi
(College of Food Science and Engineering, Inner Mongolia Agricultural University, Hohhot 010018, China)
Abstract: This study aimed to investigate the exopolysaccharide (EPS) of Lactobacillus casei HS4 and its effect on the microstructure and rheological properties of fermented milk. Two fractions of EPS, named as HS4-1-EPS and HS4-2-EPS,were obtained by size exclusion chromatography on a Sephadex G-50 column. HS4-1-EPS was mainly composed of glucose with a peak area of 0.940. HS4-2-EPS was mainly composed of glucose and mannose with a peak area ratio of 0.383:0.364.Fourier transform infrared (FT-IR) spectroscopy revealed that HS4-1-EPS and HS4-2-EPS were both heteropolysaccharides.The rheological properties and microstructure of three fermented milks made respectively with a mixed starter culture of Streptococcus thermophilus and Lactobacillus bulgaricus (1:1) in the presence and absence of the purified EPS and a monoculture of L. casei HS4 were observed to determine the effect of supplementation of the purified EPS versus in situ EPS on the rheological properties of fermented milk. These fermented milks showed different rheological properties during storage at 4 ℃. Based on the microstructure observation, we concluded that the type and space barrier effect of EPS correlated with the rheological properties of fermented milk.
Keywords: exopolysaccharide (EPS); Lactobacillus casei; structural composition; rheological properties; microstructure
Lactic acid bacteria (LAB) can produce a variety of exopolysaccharide (EPS) during its growth and metabolism.Because of its ability to combine moisture and adjust the viscosity, EPS has been widely studied as a natural stabilizer in fermented milk[1-2]. Among a series of important properties, the rheological modulation ability of EPS is of great prominence, such as thickening, stabilizing, gelling,and so on[3]. Understanding of the rheological properties of yoghurt is important in process design, product development,processing, handling, quality control and storage[4]. Results from an increasing number of recent studies have revealed that EPS were produced by different LAB strains. Among EPS-producing LAB, Lactobacillus casei is a major species of LAB[5], recent researches include L. casei LC2W[6],L. casei LC2W[7], L. casei SB27[8], and so on.
In this study, we investigated the physicochemical properties and rheological properties of EPS derived from the L. casei HS4 strain. EPS fractions were purified by Sephadex G-100 column and Sephadex G-50 column. Furthermore,their physicochemical characteristics were investigated by chemical composition detection, gas chromatographymass spectrometry (GC-MS), Fourier transform infrared(FTIR) spectroscopy. Three types of fermented milk by Streptococcus thermophilus-Lactobacillus bulgaricus(1:1), L. casei HS4, S. thermophilus-L. bulgaricus (1:1)and supplemented with purified EPS, respectively were tested as samples to determine the effect of supplemented purified EPS and the in situ EPS on rheological properties of fermented milk.
1 Materials and Methods
1.1 Bacterial strain and media
S. thermophilus and L. bulgaricus were purchased from Inner Mongolia Shuangqi Pharmaceutical Co. Ltd. (Inner Mongolia, China). The EPS-producing strain HS4 used in this study was isolated from traditionally fermented milk in Xilingol League of Inner Mongolia. The strain HS4 was identified as L. casei[9]. The strain HS4 was maintained as frozen (-80 ℃) stocks in MRS broth supplemented with 20%(V/V) glycerol[10]. They were sub-cultured twice before use.
The sterile MRS medium contained (per 100 mL): 1 g of tryptone (Sigma), 0.5 g of yeast extraction (Sigma), 1 g of beef extract (Sigma), 0.2 g of ammonium citrate, 0.5 g of sodium acetate, 1 g of MgSO4·7H2O, 0.2 g of MnSO4, 0.2 g of K2HPO4, 2 g of glucose, and 0.1 mL of Tween 80, adjusted to pH 6.8 with 1 mol/L acetic acid.
1.2 Instruments and equipment
S-530 Scanning electron microscopy (Hitachi, Tokyo,Japan); HP-5 19091J-413 GC-MS (Agilent Technologies,USA); Flame ionization detector and a DB-WAX column(30 m × 0.25 mm, 320 μm) (J&W Scientific Inc., USA);FT-IR spectrometer instrument (PerkinElmer, Inc., USA);Haake RS6000 Rheometer, CC26 Ti spindle (Thermo Fisher Scientific Inc., USA).
1.3 Methods
1.3.1 Extraction, isolation and purification of EPS
3% (V/V) of overnight culture containing about 108CFU/mL grown in the MRS medium was used as an inoculum and the cultivation was incubated in 500 mL Erlenmeyer flasks containing 300 mL of the MRS medium at 30 ℃ for 24 h.
After incubation, the EPS was extracted as described[11-12]. The culture medium was separated by centrifugation (5 000 r/min, 15 min), and the supernatant was mixed with quadruple volume chilled 95% ethanol and kept at 4 ℃ for overnight. Precipitated EPS was separated by centrifugation at 10 000 × g for 30 min at 4 ℃. The culture medium was separated by centrifugation (9 000 r/min,8 min). The supernatant was concentrated and treated with Sevag reagent (chloroform:n-butanol at 4:1, V/V) for twice to remove free protein. Total carbohydrate content and protein content of crude EPS were determined by the phenolsulphuric acid method and Coomassie Brilliant Blue method.After centrifugation, the solution was precipitated with four volumes of 95% (V/V) cold ethanol overnight at 4 ℃. The crude EPS was collected via centrifugation (10 000 r/min,10min) and dissolved in distilled water (50 g/100 mL). The supernatant was dialyzed by using a dialysis bag (8-10 kDa)in the distilled water at 4 ℃ for 48 h.
The crude EPS solution polysaccharide (1.5 mL)was fractionated with a Sephadex G-l00 column (1 cm ×50 cm), eluted with deionized water, and allow solvent to flow through the columns by gravity. Eluent was collected automatically every 5 min and the carbohydrate content was determined by phenol-sulfuric acid method. Peak fractions containing polysaccharides were pooled, dialyzed, and lyophilized. Further purification of the EPS was performed by a Sephadex G-50 column (1 cm × 50 cm) and eluted with deionized water. Eluent was collected every 5 min.The polysaccharide fractions were detected, pooled,dialyzed, and lyophilized. After that, the solution was dried by vacuum at 40 ℃, and then the powder was used for further EPS analysis.
1.3.2 Determination of monosaccharide compositions of EPS
Monosaccharide compositions of different purified EPS fractions were determined by gas chromatography (GC). A total of 2 mg of the purified EPS were hydrolyzed with 1 mL(2 mol/L) trifluoroacetic acid (TFA) at 120 ℃ for 2 h. After that, the samples were cooled down with liquid nitrogen until they were totally dried. The hydrolysate was repeatedly co-concentrated with 2 mL of methanol to dryness and converted to its trimethylsilyl (TMS) derivative. The TMS derivative of hydrolysate was prepared by adding 0.2 mL of trimethylchlorosilane, 0.4 mL of hexamethyldisilane and 1 mL of pyridine and heating at 80 ℃ for 30 min. Seven standard sugars (rhamnose, fucose, arabinose, xylose,mannose, glucose and galactose) and internal standard(inositol) were converted to their TMS derivatives in the same way. The hydrolysates were then subjected to GC-MS analysis. GC-MS was performed on an Agilent HP-5 19091J-413 fitted with a flame ionization detector and a DB-WAX column (30 m × 0.25 mm, 320 ?m).
The following chromatographic conditions were used:He was used as the carrier gas at a flow rate of 1 mL/min; the temperature of injector and detector were set at 250 ℃ and 250 ℃, respectively; initial column temperature was held at 120 ℃ for 5 min, then programmed at a rate of 3 ℃/min to 250 ℃ for 5 min. The molar ratio of the monosaccharide was calculated on the basis of the peak area of the monosaccharide.
1.3.3 FTIR spectral analysis of EPS
The major structural groups of the purified EPS were detected using FTIR spectroscopy, and the spectrum of the EPS was obtained using a KBr method[13]. The polysaccharide samples were pressed into KBr pellets at sample: KBr ratio 1:200. The FTIR were recorded on a PerkinElmer FT-IR spectrometer instrument in the region of 4 000-400 cm-1.FTIR spectrum was determined in transmission mode and the number of scans was 32. The infrared spectral resolution was 4 cm-1.
1.3.4 Preparation of fermented milk for scanning electron microscopy (SEM)
The samples particles less than 1 cm3were fixed in a 2.5% (pH 6.8) glutaraldehyde solution for 3 h at 4 ℃. The fixed samples were rinsed three times in pH 7.2 phosphate buffer (10 min every time), and dehydrated in a graded alcohol series at 30%, 50%, 70%, 80%, 90%, 95%, and 100% (15 min each gradient), and 100% for two times.The dehydrated samples were placed in isoamyl acetate for 20 min[14]. After critical point drying with CO2liquide, the thin sections were cut. This was followed by sputter coating. Finally,the prepared samples were analyzed by SEM at 20 kV.
1.3.5 Rheological properties of EPS
We detected rheological characterization by modified method[15]. Rheological characterization of fermented milks was carried out in a Haake RS6000 rheometer in rotational and oscillational modes. Rotational analysis was performed on a cup with a CC26 Ti spindle. The temperature of the system was set at 20 ℃. Shear stress was determined as a function of shear rate. Shear rate increase from 0 r/min to 300 r/min and the same but negative acceleration value to decrease shear rate until 0. The linear viscoelastic region was determined through stress sweep test at a fixed frequency(1 Hz). G’ (storage modulus) and G” (loss modulus) were evaluated at a function of strain (0.01%-100%) within the linear range. The temperature ramp test was carried out to determine the relationship between viscoelastic modulus and temperature. The test was performed at a control rate of 50 r/min and a heating rate of 6 ℃/min from 4 ℃ to 30 ℃.
1.3.6 Manufacture of fermented milk
Low heat nonfat dry milk powder was obtained from Yili Industrial Group Co. Ltd. (Inner Mongolia, China). Milk was reconstituted by dispersing 10 g of powder in 100 mL of deionized water and stirring at room temperature for 3 h[16],adjusted pH to 6.8. Reconstituted milk was heated at 110 ℃for 15 min, cooled to 37 ℃. Before experimentation,twice a week, the single-strain cultures were propagated in sterilized skim milk in test tubes as follows: L. casei HS4 at 37 ℃ up to pH 4.7; L. bulgaricus at 37 ℃ up to pH 4.7 and S. thermophilus at 37 ℃ up to pH 4.7 and stored at 4 ℃.
Control sample: Reconstituted skim milk was inoculated with was inoculated with cultures in quantities: L. bulgaricus(1.5%) + S. thermophilus (1.5%) and incubated at 37 ℃ up to pH 4.7, stored at 4 ℃ for 5 days prior to rheological analysis.
Tested sample A: Reconstituted skim milk was inoculated with 3% HS4 culture and incubated at 37 ℃ up to pH 4.7, stored at 4 ℃ for 5 days prior to rheological analysis.
Tested sample B: Reconstituted skim milk was inoculated with cultures in quantities: L. bulgaricus (1.5%) +S. thermophilus (1.5%) and supplemented with purified HS4-1-EPS and HS4-2-EPS, incubated at 37 ℃ up to pH 4.7,and stored at 4 ℃ for 5 days prior to rheological analysis.
1.4 Statistical analysis
All data were analyzed using SPSS 16.00 statistics analysis software; figures were draw using Origin 7.5.
2 Results and Analysis
2.1 Extraction, isolation and purification of EPS
The crude EPS was obtained by ethanol precipitation of the culture supernatant of L. casei HS4, the UV-vis analysis of the EPS shows no absorption at 595 nm, indicating no protein present in the crude EPS sample, and then was separated by Sephadex G-100 column (Fig. 1). Two fractions were obtained as shown in Fig. 2. The fractions corresponding to the single elution peak of the EPS were collected, and further purified by Sephadex G-50 column. Only one fraction of the EPS was obtained, and the elution peak of the EPS was collected, concentrated, dialyzed and lyophilized to obtain the purified form of the EPS, which was used for the following analyses.
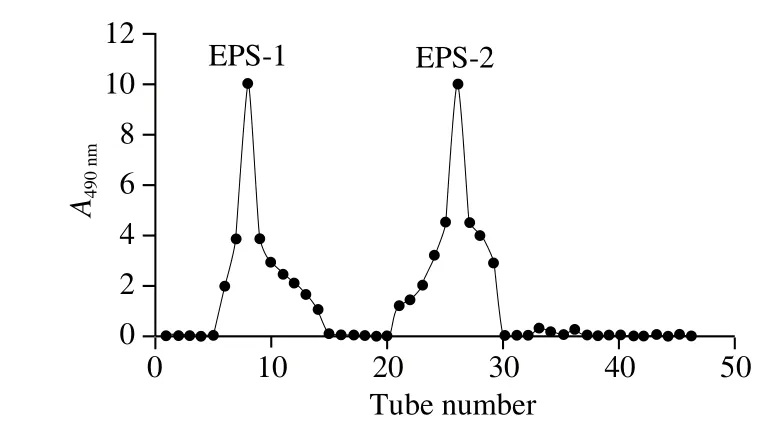
Fig. 1 Isolation of crude EPS from L. casei HS4 on Sephadex G-100 column

Fig. 2 Purification of crude EPS from L. casei HS4 on Sephadex G-50 column
2.2 Monosaccharide composition of EPS
The monosaccharide composition of HS4-1-EPS and HS4-2-EPS were determined by GC-MS, respectively and seven commercially available monosaccharides were used as standards. The result showed that HS4-1-EPS was mainly composed of glucose with the peak area ratio (close to molar ratio) of 0.940. HS4-2-EPS was composed of glucose,mannose, galactose and rhamnose with the peak area ratio of 0.383:0.364:0.135:0.062. Other monomers were present only in small amounts. These results of monosaccharide composition analysis were different with those reported in EPS from L. casei strains. The monosaccharide composition of L. casei 01 EPS was formed that the main constituent of the EPS molecules was 70% galactose and 25% glucose[17]; LCP1 from L. casei LC2W was composed of erythritol, rhamnose and glucose with the peak area ratio of 0.576:0.431:0.287[18];LW1 from L. casei SB27 was composed of 52.4% galactose,29.1% glucose and 7.2% arabinose, while LW2 contained 57.4% galactose, 22.2% glucose and 8.5% mannose[8].
2.3 FTIR analysis of HS4-EPS
FTIR spectrum was used to confirm the structural features and functional groups of polysaccharide. An EPS FTIR spectrum was performed in the region of 4 000-400 cm-1.As shown in Fig. 3, HS4-1-EPS displayed typical peaks of EPS at 3 444.00-1 033.71 cm-1, and HS4-2-EPS displayed typical peaks of EPS at 3 375.72-601.83 cm-1. A broad and intense peak around 3 400 cm-1(HS4-1-EPS: 3 444.00 cm-1;HS4-2-EPS: 3 375.72 cm-1) was attributed to the stretching vibration of hydroxyl groups (O-H)[13]. The appearance the bands at 2 927 cm-1and 2 851 cm-1is due to C-H asymmetric and symmetric stretching vibrations in the aliphatic CH2groups of HS4-1-EPS (2 852.55 cm-1and 2 923.52 cm-1)[19].In addition, the peak at 1 649 cm-1(HS4-1-EPS:1 646.39 cm-1) resulted from the stretching vibration in C=O and the carboxyl group[13]. The peak at 1 600-1 630 cm-1(HS4-2-EPS: 1 615.99 cm-1) could be conformed as antisymmetric stretching of C=O in COO- group[20]. The peak at 1 400 cm-1(HS4-1-EPS: 1 400.96 cm-1) could be due to symmetric stretching of C=O in the COO- group[21], and 1 414 cm-1(HS4-2-EPS: 1 413.54 cm-1) are assigned to stretching mode of the (CO3)2groups[19]. The peak at 1 320 cm-1(HS4-2-EPS: 1 322.75 cm-1) is conformed as ring vibration[19].The peak between 1 210 cm-1and 1 270 cm-1(HS4-2-EPS:1 271.27 cm-1) is associated with stretching vibration of the S=O bond in sulfate groups[22]. Two stretching peaks near 1 155 cm-1and 1 076 cm-1were observed for the HS4-2-EPS(1 117.89 cm-1and 1 091.32 cm-1), which suggested the presence of the glycosidic linkages C-O-C and C-OH[13].The peaks at 1 010—1 090 cm-1(HS4-2-EPS: 1 048.28 cm-1)were due to the stretching vibrations of the glycoside bridge(C-O-C)[22]. And the absorptions at 1 030 cm-1(HS4-1-EPS:1 033.71 cm-1) can be attributed to stretching of C-O and stretching of C-C[19]. Peaks around 563-875 cm-1(HS4-2-EPS: 601.83 cm-1) gives clue about the presence of saccharide moieties[23]. A comparison of functional groups revealed that the purified HS4-1-EPS and HS4-2-EPS contained different functional groups, and all were heteropolysaccharide.

Fig. 3 Infrared absorption spectra of HS4-1-EPS (A) and HS4-2-EPS (B)
2.4 Microstructure of the samples
A 3D mesh structure of the samples was observed by SEM, as shown in Fig. 4. The samples consist of a protein network and serum pores (which appear as unstained black regions). Compared with control, the casein microspheres in sample fermented by strain HS4 were closely connected with a small amount of EPS, and a small number of large pores appears. Due to the exclusive action of EPS and casein micelles, the repulsive force between casein micelles and interspaces was slightly weakened and the barrier of casein micelles was weakened by continuous and strong combination of casein micelles. Therefore, they are more difficult to be destroyed, which also explains the phenomenon of shear thinning, because the protein molecular micelles had a strong stereospecific blockade. Protein micelles gather together to form filamentous micelles with large pores in samples supplemented with purified EPS. But the voids among the structure of sample supplemented with HS4-2-EPS were larger than that with HS4-1-EPS. The results were consistent with Li Quanyang et al.[24]. With the gradual production of EPS and the interference of network, the threedimensional network structure of casein can be uniform and fine. However, when the raw milk was supplemented with purified EPS, the EPS of macromolecule completes the spatial division of raw milk before the aggregation of casein.Under strong space barrier effect, three-dimensional network structure of casein will only be formed discontinuously.The structure of fermented milk will become very weak.The coupling between the casein micelles was more tightly coupled, which increases the water holding capacity of the 3D network structure and increases the hardness of the gel. It could explain that the samples showed a different viscosity.

Fig. 4 Microstructure of fermented milk samples observed by SEM (× 5 000)
2.5 Rheological properties
Understanding of the rheological properties of fermented milk is important in processing, quality control and storage.The value of G’ is a measure of the deformation energy stored in the sample during the shear (deformation) process, which represents the elastic behavior of a sample[25]. On the contrary,the value of G” is a measure of the deformation energy used by the sample during the shear test and subsequently lost,representing the viscous behavior of a sample[26].
The average values of elastic/storage (G’), viscous/loss (G”) moduli of fermented milk made with different cultures at 1 Hz frequency are shown in Fig. 5. All fermented milks exhibited higher G’ than G” values, which means that yogurts showed more elastic than viscous behavior and were characterized as weak gels. It was in agreement with some previous results[27-28]but different with the results of other studies with some EPS producing LAB species, as they have reported lower G’ values for EPS containing fermented milk than those of control fermented milk[15]. It indicated that all types of fermented milk had elastic or solid-like character and interactions between the EPS and the protein network of the yoghurt gel. Li Quanyang et al.[24]suggested that this may be due to electrostatic interactions of polysaccharides with the caseins and whey proteins. Fermented milks supplemented with purified EPS HS4-1-EPS and HS4-2-EPS showed lower moduli than that made with L. casei HS4 and control strains(S. thermophilus and L. bulgaricus), respectively (Fig. 5).EPS interacting with proteins can act as an active filler and increase viscoelastic moduli. The presence of EPS in milk before gelation produced large pores (Fig. 4), which led to reduced viscoelastic moduli. Production of EPS after gelatin does not seem to affect the porosity of the network, but it might lead to increased rigidity[24]. Higher concentration EPS could have promoted more incompatibility with the protein network, which could also have contributed to the observed decrease in G’ values[29]. L. casei HS4 showed higher G’ than that of control fermented milk.
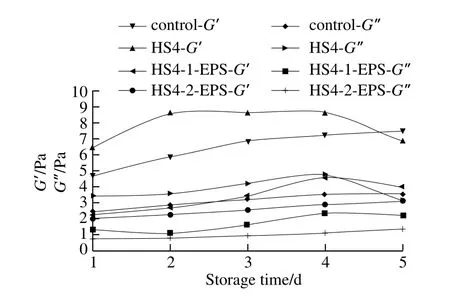
Fig. 5 Linear viscoelastic region curves of fermented milk samples
Thixotropic loops (the area between the upward and downward curve in shear stress vs. shear rate curve) is a decrease in the apparent viscosity under shearing, followed by a gradual recovery when the shear is removed. The thixotropic property can be quantified by its ability to regain its initial structure when it was allowed to rest for a period of time after disturbing[30-31]. The area of thixotropic loop of fermented milk made with S. thermophilus and L. bulgaricus was higher than that of fermented milks made with L. casei HS4, HS4-1-EPS and HS4-2-EPS, respectively (Fig. 6).This indicated that former fermented milk was subjected to more structure degradation during the upward cycle than others. Greater viscosity was strongly correlated with a greater loop area[32]. The amount and type of EPS, and their interactions and distribution in the protein network, are the factors influencing structural breakdown of the network[29].Compared with fermented milk made with L. casei HS4, the relatively higher area of thixotropic loop of fermented milks made with HS4-1-EPS and HS4-2-EPS and may indicate that EPS confer a more polymer-like rheological behavior to the continuous phase[29]. The obvious change of thixotropic loop area was not observed for all the fermented milks during storage.
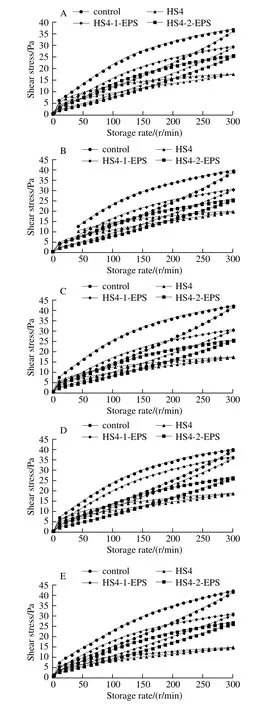
Fig. 6 Thixotropic loop of fermented milk samples during storage for 5 days
3 Conclusion
The effect of supplemented purified EPS and the in situ EPS on rheological properties of fermented milk are different.Concentration and structure of EPS determine the interactions of EPS with the protein network in fermented milk. During the formation of fermented milk gels, EPS is produced slowly by the metabolism of LAB, which is accompanied with casein agglutinating during the gel-building process.With the gradual production of EPS and the interference of network, the three-dimensional network structure of casein can be uniform and fine. However, when the raw milk was supplemented with purified EPS, the EPS of macromolecule completes the spatial division of raw milk before the aggregation of casein. Under strong space barrier effect,three-dimensional network structure of casein will only be formed discontinuously. The structure of fermented milk will become very weak. In addition, two purified EPS fractions have different effect on rheological properties of fermented milk. The preliminary structural analysis can be inferred that the composition of EPS plays a role on rheological properties.However, which specific components play important role needs further research.

