有機胺改性對ZIF-8催化Knoevenagel縮合反應活性的影響
高朋召 吳迪 鄭航博 陳會會 張佩
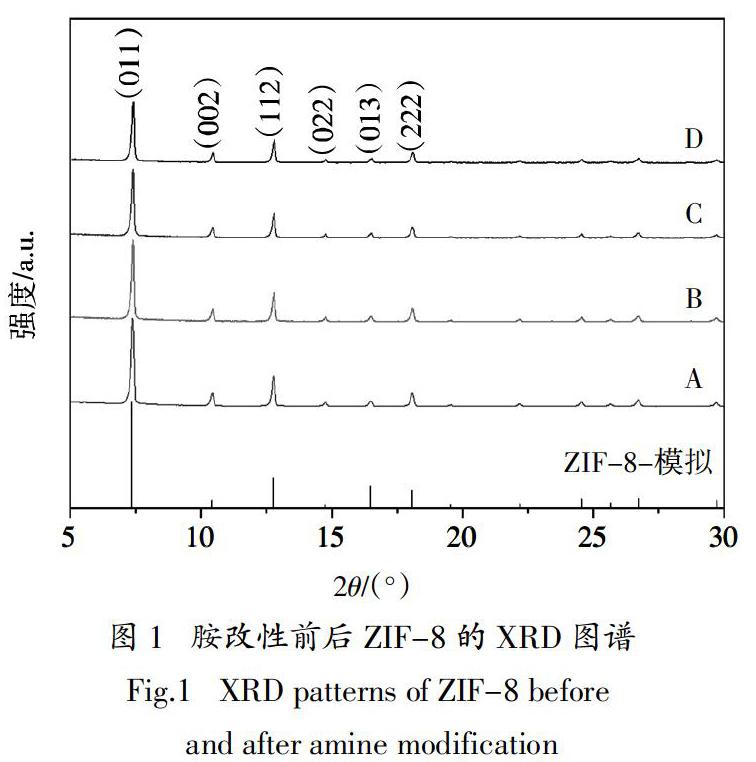
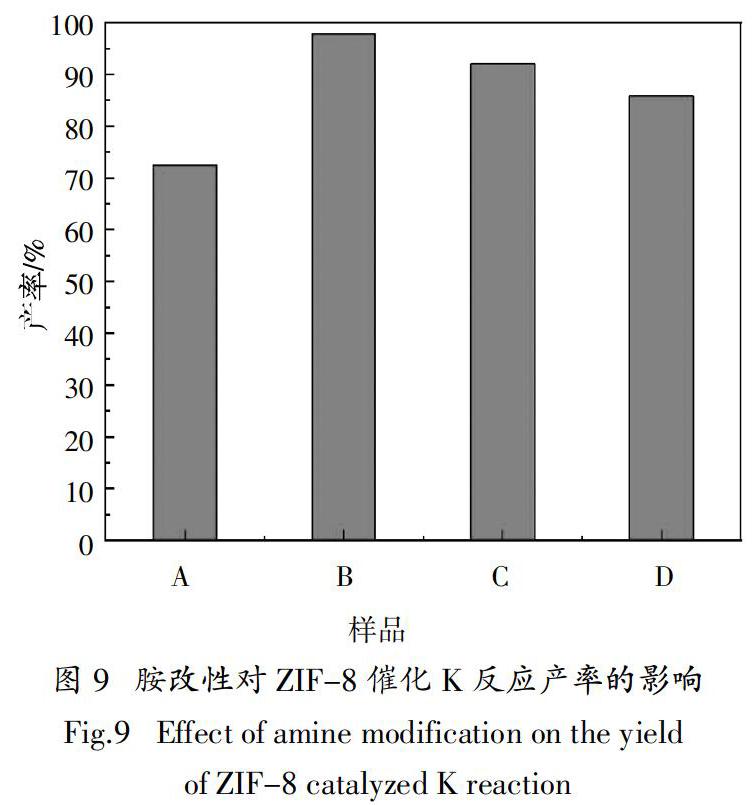
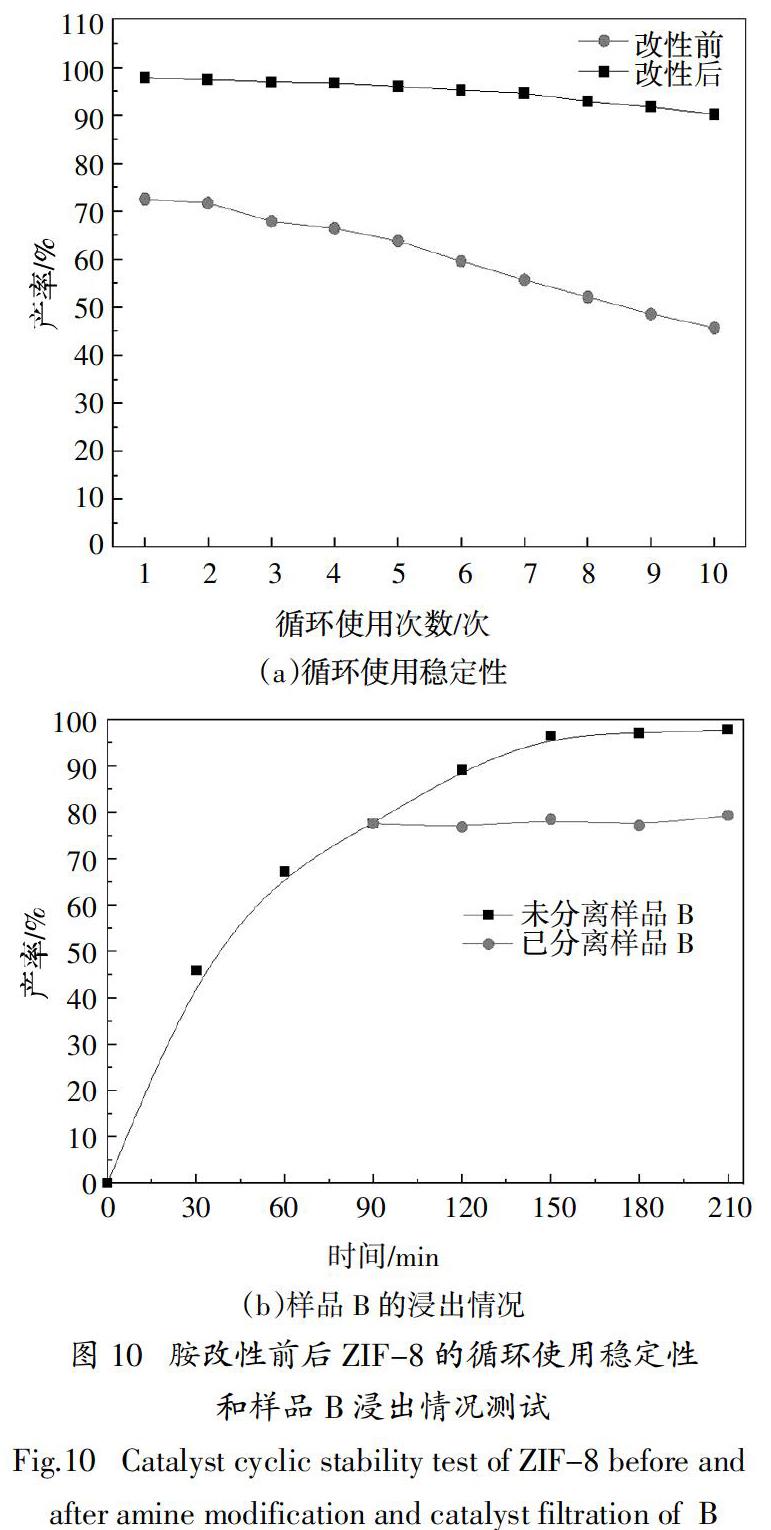
摘? ?要:采用不同結構的有機胺改性溶劑熱法合成ZIF-8催化劑,探討胺結構特性對ZIF-8催化Knoevenagel縮合反應活性的影響. 結果表明,有機胺改性后ZIF-8保持菱形十二面體結構,形貌規則與未改性材料無明顯差別,1,2丙二胺、二乙烯三胺和三乙烯四胺改性ZIF-8后的BET比表面積分別為1 893 m2·g-1、1 885 m2·g-1和1 861 m2·g-1,較改性前下降約6.5 %,這主要是由于接枝在ZIF-8表面的有機胺堵塞了其孔道;采用乙醇作溶劑,催化劑添加量(摩爾分數)為0.6%(相對于苯甲醛用量),反應溫度80 ℃,210 min時,1,2丙二胺改性ZIF-8對Knoevenagel反應的催化活性最高,α-氰基肉桂酸乙酯的產率達97.8%,循環10次后,產率依舊保持90 %以上,較未改性催化劑產率提高35.3 %. 對胺改性ZIF-8的催化機理研究表明:有機胺改性ZIF-8可增加其催化活性位點,而1,2丙二胺因鏈短,空間位阻小,其N活性位點更易與反應物接觸,與ZIF-8上原有的咪唑N位點一起通過孤對電子與反應物苯甲醛的亞甲基上的 -H配位,從而顯著提高催化劑的活性.
關鍵詞:ZIF-8;Knoevenagel縮合反應;胺改性;機理;催化
中圖分類號:O641? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? ? 文獻標志碼:A? ?文章編號:1674—2974(2020)08—0124—09
Abstract:In this paper, different kinds of organic amines were used to modify ZIF-8 catalysts prepared via solvothermal method,and the effects of organic amine structural characteristics on the catalytic activity of ZIF-8 for Knoevenagel condensation reaction were discussed. The results show that ZIF-8 modified by organic amine still maintains the rhombohedral dodecahedron structure and possesses regular morphology,without obvious difference from that of unmodified materials. The BET specific surface areas of ZIF-8 modified by 1,2-propylenediamine,diethylenetriamine and triethylenetetramine are 1 893 m2·g-1,1 885 m2·g-1 and 1 861 m2·g-1,respectively, decreased by about 6.5% compared with that of the unmodified materials, mainly due to the blockage of pores for ZIF-8 by organic amines. When ethanol works as solvent, the amount of catalyst is 0.6 mol%(molar ratio to benzaldehyde),the reaction temperature is 80 ℃. When the reaction time keeps for 210 min,1,2-propanediamine modified ZIF-8 exhibits the highest catalyst activity,and the yield of ethyl α-cyanocinnamate reaches 97.8 %. After 10 cycles,the yield of this reaction is still up to 90 %,an increase of 35.3 % higher than that of unmodified ZIF-8. The studies on the catalytic mechanism of amine-modified ZIF-8 indicate that amines modified ZIF-8 can improve the amount of activity site,while 1,2-propanediamine has a shorter chain and less steric hindrance, making its N sites easier contact with reactants. It combines with the original imidazole N site on ZIF-8 to coordinate with α-H on the methylene of benzaldehyde through lone pair electrons,? therefore significantly improving the catalytic activity of the catalyst clearly.
Key words:ZIF-8;Knoevenagel condensation reaction;amine modification;mechanism;catalysis
金屬有機骨架材料MOFs具有結構多樣、孔隙率高、表面性質可調和易實現功能化等優勢,在吸附/分離、多相催化、化學傳感、藥物運輸、光電材料、氣體儲存等領域實現了廣泛應用[1]. 在MOFs材料合成過程中,由于空間位阻效應,金屬離子與有機配體不完全配位,為滿足其配位穩定性需要,金屬離子還會與一些溶劑小分子如水、甲醇等發生弱相互作用. 將其在高溫真空環境處理后,這些小分子由于弱的相互作用會脫離骨架,導致金屬離子無法實現飽和配位變成缺陷[2],這些位置給胺改性提供了接枝位點.
Knoevenagel反應(簡稱K反應)是指吡啶、哌啶和胺等弱堿性催化劑催化醛或酮與帶有活潑亞甲基(α-H)的有機物反應,是精細化工合成中最基本的縮合反應之一[3]. 目前關于胺改性MOFs用于催化K反應取得了一定的進展. Huang等[4]研究發現,胺改性得到的NH2-Tb-MOF對K反應的催化活性與均相催化劑苯胺相當(86%),同時對反應底物表現出一定的尺寸選擇性;Ren等[5]利用乙二胺改性的鑭系MOFs作為K反應的催化劑,效率達到了99 %以上,且循環3次后仍能保持在96 %左右;Hwang等[6]研究發現,乙二胺改性前后MIL-101在80 ℃下對K反應催化效率分別為31.5%和 97.7 %,表明胺改性能顯著提高其催化活性.
在K反應中,含亞甲基的反應底物因分子大小不同常表現出不同的反應活性,相對于丙二腈(0.69 nm × 0.45 nm),大分子底物氰乙酸乙酯(1.03 nm × 0.58 nm)的反應活性更低,所需的反應條件更苛刻;在精細化工合成領域,氰乙酸乙酯與苯甲醛反應生成的α-氰基肉桂酸乙酯是一種重要的藥用中間體,故為該反應提供更高活性的催化劑顯得尤為重要[7]. 近年來,ZIF-8作為非均相催化劑,在K反應、環加成和Friedel-Crafts酰化等有機合成反應中均表現出良好的催化效果[8]. 通過對ZIF-8胺改性,有機胺上的一個氮原子可與Zn2+配位,而另一個氮原子作為催化K反應的活性中心,故可在一定程度上增強其催化效果[5].
目前有機胺結構對胺改性MOFs催化K反應活性的研究鮮有報道. 本文分別選擇1,2丙二胺、二乙烯三胺和三乙烯四胺作為ZIF-8的3種有機胺改性劑,探討胺的結構特性對ZIF-8催化苯甲醛和氰乙酸乙酯反應活性的影響. 改性前ZIF-8記為樣品A,丙二胺、二乙烯三胺和三乙烯四胺改性ZIF-8分別標記為樣品B、C和D.
1? ?實? ?驗
1.1? ?原料
主要的試劑有六水硝酸鋅(Zn(NO3)2·6H2O)、2-甲基咪唑(C4H6N2,2-IM)、苯甲醛(C7H6O,BA)、氰基乙酸乙酯(C5H7NO2,ECA)、1,2丙二胺(C3H10N2,AP)、二乙烯三胺(C4H13N3,DETA)、三乙烯四胺(C6H18N4,TETA)、無水乙醇、甲醇、甲苯以及色譜校準樣α-氰基肉桂酸乙酯(ECPA),均為分析純.
1.2? ?ZIF-8制備工藝及胺改性
ZIF-8的合成工藝參照文獻[9],進行了部分修改:將Zn(NO3)2·6H2O(3 mmol)與2-甲基咪唑(12 mmol)分別溶解在30 mL和20 mL無水甲醇中,固體完全溶解后,將Zn鹽溶液在攪拌下迅速加入咪唑溶液中,攪拌5 min后將混合物轉移到100 mL聚四氟反應釜中,密封,在140 ℃保溫24 h. 冷卻至室溫后從混合物中除去母液,用無水甲醇離心洗滌3~5次,在80 ℃下隔夜干燥后備用.
根據Miralda等[10]提供的方案并修改后進行胺改性ZIF-8實驗:將得到的ZIF-8粉體在100 ℃干燥24 h進行預活化,取200 mg ZIF-8懸浮在30 mL甲苯中,分別加入0.1 mmol 1,2丙二胺、二乙烯三胺和三乙烯四胺,85 ℃回流20 h,冷卻后的產物用甲醇徹底洗滌,并在85 ℃真空干燥24 h.
1.3? ?測試與表征
采用X射線衍射儀(XRD,Rigaku D/max2200)對胺改性前后的ZIF-8進行物相分析. 測試條件:Cu-Ka射線,掃描范圍10° ~ 80°,步長0.02,掃描速度為8°/min. 采用JSM-6700場發射掃描電子顯微鏡對胺改性前后的ZIF-8進行微觀形貌觀察. 采用FT-IR(Perkin Elmer Spectrum One)對胺改性前后的ZIF-8中存在的官能團進行分析. 操作條件為KBr壓片,波長范圍為4 000~400 cm-1. 采用美國物理電子公司的PEI5700型X-射線光電子能譜儀進行元素分析. 采用德國耐馳公司的STA-449C綜合熱分析儀研究胺改性前后的ZIF-8的熱穩定性,操作條件為空氣中,以5 ℃·min-1的升溫速率測量從室溫到800 ℃. 氮氣吸附-脫附測試借助ASAP2020全自動比表面積及孔隙率分析儀完成,樣品測試前在100 ℃條件下真空干燥24 h,測試時脫氣條件設為120 ℃、24 h,比表面積和孔徑分別由BET公式和BJH方法計算得到.
1.4? ?催化劑活性及循環穩定性測試
催化劑活性測試:K反應在裝有回流冷凝器的磁性攪拌圓底燒瓶中進行. 將1.0 mL苯甲醛、1.1 mL氰基乙酸乙酯和2.9 mL無水乙醇組成的反應混合物加入燒瓶后,在反應體系中加入一定量胺改性前后的ZIF-8,水浴加熱,同時通入氮氣并緩慢攪拌,考察因素分別為溫度、催化劑用量以及胺結構特性,使用前所有催化劑過400目篩. 反應過程中定時取樣收集,并通過氣相色譜儀GC7890B檢測,色譜柱采用HP-5型毛細石英管柱,規格為30 m×320 μm×0.25 μm,檢測器采用氫火焰離子化檢測器(FID),通過不同濃度的純α-氰基肉桂酸乙酯建立標準曲線來計算反應的產率[11].
[13]? LIU B,JIAN M,LIU R,et al. Highly efficient removal of arsenic(III) from aqueous solution by zeolitic imidazolate frameworks with different morphology[J]. Colloids and Surfaces a Physicochemical and Engineering Aspects,2015,481:358—366.
[14]? DEVI S,SINGH B,PAUL A K,et al. Highly sensitive and selective detection of trinitrotoluene using cysteine-capped? gold nanoparticles[J].Analytical Methods,2016,8:4398—4405.
[15]? ORDONEZ M J C,BALKUS K J,FERRARIS J P,et al. Molecular sieving realized with ZIF-8/matrimid@ mixed-matrix membranes [J]. Journal of Membrane Science,2010,361(1/2):28—37.
[16]? REN H,ZHANG L,AN J,et al. Polyacrylic acid@zeolitic imidazolate framework-8 nanoparticles with ultrahigh drug loading capability for pH-sensitive drug release[J]. Chemical Communications,2013,50(8):1000—1002.
[17]? WANG Z,WANG D,ZHANG S,et al. Interfacial design of mixed matrix membranes for improved gas separation performance[J]. Advanced Materials,2016,28(17):3399—3405.
[18]? LIU J,HE J,WANG L Y,et al. NiO-PTA supported on ZIF-8 as a highly effective catalyst for hydrocracking of Jatropha oil[R]. Scientific Reports,2016:23667.
[19]? MICHAEL C,CECILIA C,STEPHANIE M A,et al. Rhodamine B degradation by nanosized zeolitic imidazolate framework-8 (ZIF-8) [J]. RSC Advances,2018,47(8):26987—26997.
[20]? WU J B,LIN Y F,WANG J,et al. Correlation between N 1s XPS binding energy and bond distance in metal amido,imido,and nitrido complexes[J]. Inorganic Chemistry,2003,42(15):4516—4518.
[21]? WU Y N,ZHOU M M,ZHANG B R,et al. Amino acid assisted templating synthesis of hierarchical zeolitic imidazolate-framework-8 for efficient arsenate removal[J]. Nanoscale,2014,6:1105—1112.
[22]? REICHENBACH C,KALIES G,ENKE D ,et al. Cavitation and Pore blocking in nanoporous glasses[J]. Langmuir,2011,27(17):10699—10704.
[23]? DONG X,SU Y,LU T,et al. MOFs-derived dodecahedra porous Co3O4:an efficient cataluminescence sensing material for H2S[J]. Sensors and Actuators B:Chemical,2018,258:349—357.
[24]? XIA J,DIAO K,ZHENG Z,et al. Porous Au/ZnO nanoparticles synthesised through a metal organic framework (MOF) route for enhanced acetone gas-sensing[J]. RSC Advances. 2017,61(7):38444—38451.
[25]? SCHIJNDEL V,CANALLE J A M,MOLENDIJK L A,et al. The green Knoevenagel condensation:solvent-free condensation of benzaldehydes[J]. Green Chemistry Letters and Reviews,2017,10(4):404—411.
[26]? TROTZKI R,HOFFMANN M M,ONDRUSCHKA B. Studies on the solvent-free and waste-free Knoevenagel condensation[J]. Green Chemistry,2008,10(7):767—772.
[27]? TRAN U P N,LE K K A,PHAN N T S. Expanding applications of metal-organic frameworks:zeolite imidazolate framework ZIF-8 as an efficient heterogeneous catalyst for the Knoevenagel reaction[J]. ACS Catalysis,2011,1(2):120—127.
[28]? ZHAO Y,DING H,ZHONG Q. Preparation and characterization of aminated graphite oxide for CO2 capture[J]. Applied Surface Science,2012,258(10):0—4307.
[29]? ZHANG P,XIAO Y,SUN H,et al. Microwave-assisted,Ni-induced fabrication of hollow ZIF-8 Nanoframes for Knoevenagel reaction [J]. Crystal Growth & Design,2018,18(7):3841—3850.

