胃癌患者術后預后的獨立危險因素及淋巴結轉移率對不同pN分期胃癌患者預后的影響
余協媛 徐永燦 汪偉民

[摘要] 目的 分析胃癌患者術后預后的獨立危險因素及淋巴結轉移率對不同pN分期胃癌患者預后的影響。 方法 隨機選取2014年1月至2015年10月在湖州市中心醫院進行手術切除治療的胃癌患者100例作為研究對象。采用電話、門診等方法進行隨訪,隨訪時間超過60個月。比較100例胃癌患者的臨床基本資料,采用Log-Rank檢驗分析100例胃癌患者60個月生存率變化。采用Kaplan-Meier檢驗分析影響胃癌患者術后預后的單因素。采用Cox比例風險回歸模型分析影響胃癌患者術后預后的多因素。采用分層分析淋巴結轉移率對不同pN分期胃癌患者預后的影響。 結果 100例胃癌患者中,男性比例明顯高于女性,年齡大多超過55歲,腫瘤直徑<4 cm,腫瘤部位以胃下多見,pT4分期占比明顯高于pT1、pT2、pT3,pN0、pN1、pN2、pN3分期占比分別為32.00%、20.00%、22.00%、26.00%;淋巴結轉移率,無轉移、≤20%、≥21%占比分別為32.00%、18.00%、50.00%,患者CA19-9、CEA含量分別以低于27 U/ml、5 μg/L多見,血小板計數在(100~300)×109個/L,胃癌大多數為低分化,且患者術后經化療治療。100例胃癌患者中,術后12、36、60個月生存率分別為80.00%、60.00%、52.00%。經Kaplan-Meier檢驗分析,腫瘤直徑、腫瘤部位、pT分期、pN分期、淋巴結轉移率、CA19-9、CEA、血小板計數、原發術后化療、分化程度均為影響胃癌預后的危險因素(P<0.05)。經Cox比例風險回歸模型分析,腫瘤直徑、pT分期、pN分期、淋巴結轉移率、CA19-9、CEA等均為影響胃癌患者術后預后的獨立危險因素(P<0.05)。經Kaplan-Meier分析,淋巴結轉移率對pN1期及pN2期胃癌患者的60個月生存率具有明顯影響(P<0.05)。 結論 腫瘤直徑、pT分期、pN分期、淋巴結轉移率、CA19-9、CEA均為胃癌患者術后預后的獨立危險因素,淋巴結轉移率對pN1、pN2胃癌患者60個月生存率有顯著影響。
[關鍵詞] 胃癌;預后;獨立危險因素;淋巴結轉移率;pN分期
[中圖分類號] R735.2? ? ? ? ? [文獻標識碼] A? ? ? ? ? [文章編號] 1673-9701(2022)01-0008-06
The influence of independent risk factors for the prognosis of gastric cancer patients and lymph node metastasis rate on the prognosis of gastric cancer patients with different pN stages
YU Xieyuan1? ?XU Yongcan1? ?WANG Weimin2
1.Department of Surgery, Huzhou Central Hospital,Affiliated Central Hospital of Huzhou Normal College, Huzhou 313000, China; 2.Department of Gastrointestinal Surgery, Huzhou Central Hospital, Affiliated Central Hospital of Huzhou Normal College, Huzhou? ?313000, China
[Abstract] Objective To analyze the effect of independent risk factors for the prognosis of gastric cancer patients and lymph node metastasis rate on the prognosis of gastric cancer patients with different pN stages. Methods A total of 100 cases of gastric cancer patients who underwent surgical resection in Huzhou Central Hospital from January 2014 to October 2015 were randomly selected as the research objects. Follow-up was conducted by telephone and outpatient, and the follow-up time was more than 60 months. The basic clinical data of 100 patients with gastric cancer were compared, and the 60-month survival rate of 100 patients with gastric cancer was analyzed by Log-Rank test. The Kaplan-Meier test was used to analyze the single factors affecting the prognosis of gastric cancer patients after surgery. The Cox proportional hazards regression model was used to analyze the multiple factors affecting the prognosis of gastric cancer patients after surgery. The effect of lymph node metastasis rate on the prognosis of gastric cancer patients with different pN stages was analyzed by stratification analysis. Results Among 100 patients with gastric cancer, the proportion of males was significantly higher than that of females. Most of them were over 55 years old. The tumor diameter was less than 4 cm. The tumor sites were more common under the stomach. The proportion of pT4 staging was significantly higher than that of pT1, pT2, pT3. The proportions of pN0, pN1, pN2 and pN3 stages were 32.00%, 20.00%, 22.00%, and 26.00%, respectively. The proportions of lymph node metastasis without metastasis, ≤20%, and ≥21% were 32.00%, 18.00%, and 50.00%, respectively.It was more common to be lower than 27 U/ml and 5 μg/L in patients with CA19-9 and CEA contents. The platelet count was about (100-300)×109/L. The majority of gastric cancer was poorly differentiated, and the patients were treated with chemotherapy after surgery. Among 100 patients with gastric cancer, the survival rates at 12 months, 36 months, and 60 months after surgery were 80.00%, 60.00%, and 52.00%, respectively.According to Kaplan-Meier test analysis, tumor diameter, tumor location, pT stage, pN stage,lymph node metastasis rate, CA19-9, CEA, platelet count, primary postoperative chemotherapy, and degree of differentiation were all risk factors affecting the prognosis of gastric cancer(P<0.05). According to Cox proportional hazard regression model analysis,tumor diameter, pT staging, pN staging, lymph node metastasis rate, CA19-9, and CEA were all independent risk factors affecting the prognosis of gastric cancer patients after surgery (P<0.05). According to Kaplan-Meier analysis, the lymph node metastasis rate had a significant impact on the 60-month survival rate of pN1 and pN2 gastric cancer patients(P<0.05). Conclusion Tumor diameter, pT stage, pN stage, lymph node metastasis rate, CA19-9, CEA are all independent risk factors for postoperative prognosis of gastric cancer patients. Lymph node metastasis rate has a significant impact on the 60-month survival rate of pN1 and pN2 gastric cancer patients.
[Key words] Gastric cancer; Prognosis; Independent risk factors; Lymph node metastasis rate; pN staging
胃癌是一種消化道惡性腫瘤,是臨床上常見的惡性疾病之一,據統計,我國每年胃癌新增病例超90萬,而其中死亡患者超過66萬。隨著人們生活水平的提高,生活方式的改變,胃癌的發病率正逐年提高,且逐漸趨于年輕化,不僅對患者及其家庭的生活造成巨大影響,更對社會發展造成嚴重影響[1]。目前,臨床上治療胃癌大多采用手術切除聯合術后化療的綜合治療方法,并取得重大進步,但患者5年生存率仍較低。有研究發現[2-3],腫瘤部位、浸潤程度、淋巴結轉移之間可相互聯系、相互作用,共同影響患者的預后,其中,淋巴結轉移是胃癌的主要轉移方式,也是影響胃癌患者生存質量的重要因素。有研究證實,在一定范圍內,檢測出的淋巴結個數越多,患者的生存時間越長,預后越好[4]。此外,患者的淋巴結轉移個數與胃癌的pT分期明顯相關,對于不同分期的患者,淋巴結轉移范圍及個數也不盡相同[5]。但關于淋巴結轉移個數與pN分期的關系尚不明確。本研究主要對胃癌患者術后預后的獨立危險因素及淋巴結轉移率對不同pN分期胃癌患者預后的影響進行研究,現報道如下。
1 資料與方法
1.1 一般資料
隨機選取2014年1月至2015年10月在湖州市中心醫院進行手術切除治療的胃癌患者100例作為研究對象。納入標準:①符合胃癌的診斷及治療[6],且經病理學檢查確診者;②無淋巴結或鄰近器官轉移者;③參與研究前未經放化療治療者;④本組研究經本院醫學倫理委員會同意批準,均符合醫學倫理學;⑤本人及家屬均知情同意并簽訂知情同意書。排除標準:①病歷資料不全或中途退出者;②伴有乙肝等感染性疾病患者;③行單純性改道手術治療或姑息性手術者。
1.2 隨訪
采用電話、門診等方法進行隨訪,隨訪時間為60個月。
1.3 方法
基本信息:根據年齡是否超過55歲分為>55歲55例及≤55歲45例;根據性別分為男77例及女23例。
手術情況:根據腫瘤部位分為胃上18例、胃中27例、胃下53例及全胃2例;根據術后是否化療分為有化療76例,無化療24例。
病理情況:根據腫瘤直徑是否超過4 cm分為>4 cm 44例及≤4 cm 56例。根據侵犯程度分為pT1期(腫瘤侵犯黏膜下層)12例、pT2期(腫瘤侵犯固有肌層)17例、pT3期(腫瘤侵犯漿膜下層)8例及pT4期(侵犯指臟層腹膜、鄰近組織或結構)]63例。根據淋巴結轉移成都分為pN0期(無陽性淋巴結轉移)32例、pN1期(淋巴結轉移1~2枚)20例、pN2期(淋巴結轉移3~6枚)22例、pN3期(淋巴結轉移超過7枚)26例。根據淋巴結轉移率分為無轉移32例、轉移率≤21% 18例、轉移率≥21% 50例。根據組織學分化程度分為低分化56例、中分化25例、高分化19例。
生化資料:根據糖類抗原19-9(carbohydrate antigen 19-9,CA19-9)表達水平分為>27 U/ml 26例,≤27 U/ml 74例;根據癌胚抗原(carcinoembryonic antigen,CEA)表達水平分為>5 μg/L 28例,≤27 μg/L 72例;根據血小板計數分為>300×109個/L 13例,(100~300)×109個/L 81例,<100×109個/L 6例。
1.4 觀察指標
①100例胃癌患者的臨床基本資料,隨訪12、24、60個月時100例胃癌患者的生存率變化,影響胃癌患者術后預后的單因素及多因素分析,淋巴結轉移率對不同pN分期胃癌患者預后的影響。②采用Log-Rank檢驗分析100例胃癌患者60個月生存率變化。③采用Kaplan-Meier檢驗分析影響胃癌患者術后預后的單因素。④采用Cox比例風險回歸模型分析影響胃癌患者術后預后的多因素。
1.5 統計學方法
采用SPSS 19.0統計學軟件包進行數據分析。生存率差異比較采用Log-Rank檢驗分析。單因素分析采用Kaplan-Meier檢驗分析,多因素分析采用Cox比例風險回歸模型分析。淋巴結轉移率對不同pN分期胃癌患者預后的影響采用分層分析。P<0.05為差異有統計學意義。
2 結果
2.1 胃癌患者臨床資料分析
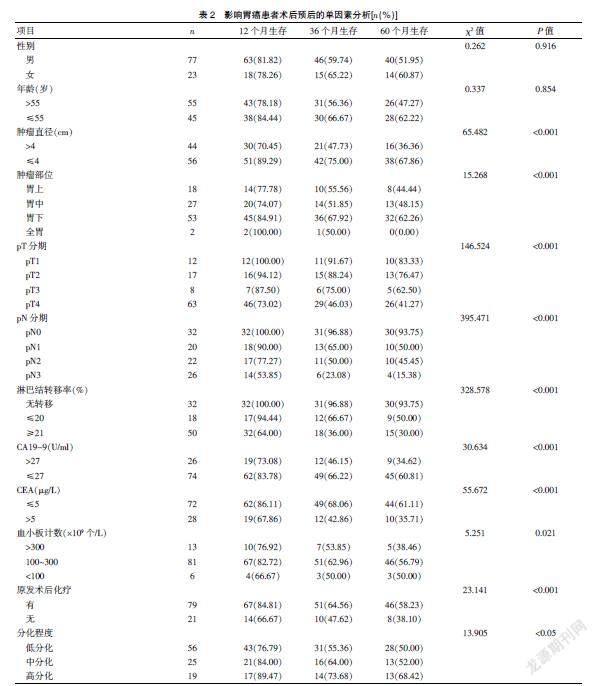
100例胃癌患者中,男性比例明顯高于女性,年齡大多超過55歲,腫瘤直徑<4 cm,腫瘤部位以胃下多見,pT4分期占比明顯高于pT1、pT2、pT3,pN0、pN1、pN2、pN3分期占比分別為32.00%、20.00%、22.00%、26.00%;淋巴結轉移率無轉移、≤20%、≥21%占比分別為32.00%、18.00%、50.00%;患者CA19-9、CEA含量分別以低于27 U/ml、5 μg/L多見,血小板計數在(100~300)×109個/L左右,胃癌大多數為低分化,且患者術后經化療治療。見表1。
2.2 影響胃癌患者術后預后的單因素分析
100例胃癌患者中,術后12、36、60個月生存率分別為80.00%、60.00%、52.00%。經Kaplan-Meier檢驗分析,腫瘤直徑、腫瘤部位、pT分期、pN分期、淋巴結轉移率、CA19-9、CEA、血小板計數、原發術后化療、分化程度均為影響胃癌預后的危險因素(P<0.05)。見表2。
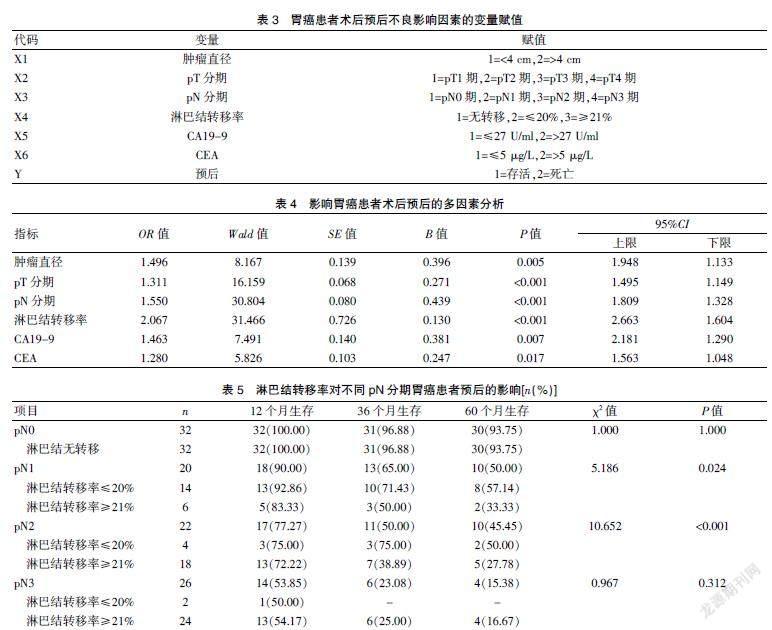
2.3 影響胃癌患者術后預后的多因素分析
經Cox比例風險回歸模型分析,腫瘤直徑、pT分期、pN分期、淋巴結轉移率、CA19-9、CEA等均為影響胃癌患者術后預后的獨立危險因素(P<0.05)。見表4。
2.4 淋巴結轉移率對不同pN分期胃癌患者預后的影響
經Kaplan-Meier分析,淋巴結轉移率對pN1期及pN2期胃癌患者的60個月生存率具有明顯影響(P<0.05)。見表5。
3 討論
胃癌是臨床上最常見的惡性腫瘤之一,據統計,在世界范圍內,每年胃癌患者的新發病人數超過100萬,而死亡人數超過75萬,在全球腫瘤相關性死亡疾病中居于第二位[7]。手術治療是臨床上治療胃癌的主要方法,但術后胃癌復發率較高,而對于胃癌復發尚無特異性有效診斷方法[8]。隨著醫學科技的發展,醫療設備的完善,臨床上診斷及治療胃癌取得重大進展,但患者的死亡率仍居高不下,且患者預后較差[9]。有研究認為[10],胃癌的發生是一個多因素參與的過程,多種因素參與其中,協同作用。
相關統計,超過50%的癌癥患者會并發貧血[11]。血小板是血細胞中最小的細胞,在止血、傷口愈合、炎癥反應及器官移植排斥等過程中扮演著重要角色[12]。Cao等[13]研究發現,血小板能阻止腫瘤細胞溶解,屏蔽腫瘤細胞表面和逃逸系統,促進細胞轉移,從而對腫瘤細胞起到一定的保護作用。血小板計數是評價血小板數量的有效指標。本研究結果顯示,血小板計數是影響胃癌患者術后預后的重要危險因素。
CA19-9、CEA均為相關腫瘤標記物,正常情況下,其表達量極低,而當發生腫瘤時,其表達水平明顯升高[14]。Shufang等[15]研究發現,CA19-9、CEA表達水平與患者淋巴結轉移及生存率高低明顯相關,與本研究結果相似。本研究結果顯示,CA19-9、CEA表達水平越高,患者的60個月生存率越低,且其均為影響胃癌患者術后的獨立危險因素。
據報道,胃癌患者原發性腫瘤的臨床病理特征及術后TNM分期與胃癌術后復發密切相關[16]。但由于各種因素的局限性和差異性,國內外對胃癌根治術后早期復發的獨立危險因素的研究結果并不一致[17]。本研究結果發現,腫瘤直徑、pT分期、pN分期、淋巴結轉移率、分化程度均為影響胃癌預后的危險因素。腫瘤分期系統是為了評估腫瘤疾病的預后、治療及結果等而開發的分類系統,JGCA與UICC/AJCC臨床上常見的評估胃癌患者預后的重要方法,而由于淋巴結轉移是胃癌最主要的轉移方式[18],因此,胃癌的淋巴結狀況被認為是最能影響胃癌患者預后的獨立危險因素。有研究發現,淋巴結轉移率能夠明顯減少階段遷移現象,其對患者的預后具有重要的預測能力[19]。本研究結果表明,腫瘤直徑、pT分期、pN分期、淋巴結轉移率等均為影響胃癌患者術后預后的獨立危險因素。淋巴結轉移率不僅是胃癌的獨立危險因素,且較pN分期具有更獨特的優勢,胃癌淋巴結轉移的存在和程度直接影響胃癌患者的預后情況[20]。淋巴結轉移率對pN1期及pN2期胃癌患者的60個月生存率具有明顯影響。
綜上所述,腫瘤直徑、pT分期、pN分期、淋巴結轉移率、CA19-9、CEA均為胃癌患者術后預后的獨立危險因素,淋巴結轉移率對pN1、pN2胃癌患者60個月生存率有顯著影響。
[參考文獻]
[1] Logan RP.Helicobacter pylori and gastric cancer[J]. Lancet(London,England),2019,344(8929):1078-1079.
[2] Spolverato G,Pawlik TM.Clinicopathological evaluation of recurrence in early gastric cancer[J].American Journal of Surgery,2019,157(3):202-207.
[3] Bai T,Yokobori T,Altan B,et al.High STMN1 level is associated with chemo-resistance and poor prognosis in gastric cancer patients[J].British Journal of Cancer,2017, 116(9):1177-1185.
[4] Kino H,Nakano M,Kanamori A,et al.Gastric adenocarcinoma of the fundic gland type after endoscopic therapy for metachronous gastric cancer[J]. Internal Medicine,2018,57(6):795-800.
[5] Katsuyuki,Murai,Kohei,et al.Effect of double-layer structure in intramucosal gastric signet-ring cell carcinoma on lymph node metastasis:A retrospective,single-center study[J].Gastric Cancer,2019,22(4):751-758.
[6] 鄭振東,韓濤.胃癌診療研究進展[J].臨床軍醫雜志,2017,45(1):1-4.
[7] Zhao R,Zhang Y,Zhang X,et al. Exosomal long noncoding RNA HOTTIP as potential novel diagnostic and prognostic biomarker test for gastric cancer[J]. Molecular Cancer,2018,17(1):68.
[8] Lu Xueying,Li Yanhong,Li Xiaobo,et al. Luteolin induces apoptosis in vitro through suppressing the MAPK and PI3K signaling pathways in gastric cancer[J].Oncology Letters,2017,14(2):1993-2000.
[9] Yiren H,Yingcong Y,Sunwu Y,et al. Long noncoding RNA MALAT1 regulates autophagy associated chemoresistance via miR-23b-3p sequestration in gastric cancer[J]. Molecular Cancer,2017,16(1):174.
[10] Cheng Y,Song Y,Qu J,et al.The chemokine receptor CXCR4 and c-MET cooperatively promote epithelial-mesenchymal transition in gastric cancer cells[J]. Translational Oncology,2018,11(2):487.
[11] Virgilio E,Giarnieri E,Giovagnoli MR,et al.Gastric lavage malignant cells(yGL) and hypohemoglobinemia (yAnemia) as new systems of tumor regression grading and prognostic prediction for gastric cancer after neoadjuvant treatment[J]. Anticancer Research,2019,39(2):1019-1027.
[12] Yun ZY,Li N,Zhang X,et al. Mean platelet volume,platelet distribution width and carcinoembryonic antigen to discriminate gastric cancer from gastric ulcer[J]. Oncotarget,2017,8(37):62 600-62 605.
[13] Cao W,Yao X,Cen D,et al.The prognostic role of platelet-to-lymphocyte ratio on overall survival in gastric cancer:A systematic review and meta-analysis[J]. BMC Gastroenterology,2020,20(1):16.
[14] Shen M,Wang H,Wei K,et al.Five common tumor biomarkers and CEA for diagnosing early gastric cancer:A protocol for a network meta-analysis of diagnostic test accuracy[J].Medicine,2018, 97(19):e0577.
[15] Shufang N,Wene W,Jilin L,et al. Clinical significance and diagnostic capacity of serum TK1,CEA,CA19-9 and CA72-4 levels in gastric and colorectal cancer patients[J].Journal of Cancer,2018,9(3):494-501.
[16] Mahar AL,Jeong Y,Zagorski B,et al.Validating an algorithm to identify metastatic gastric cancer in the absence of routinely collected TNM staging data[J]. BMC Health Services Research,2018,18(1):309.
[17] Acher AW,Squires MH,Fields RC,et al.Readmission following gastric cancer resection:Risk factors and survival[J].Journal of Gastrointestinal Surgery,2016,20(7):1-11.
[18] Ikoma N,Estrella JS,Blum M,et al.Central lymph node metastasis in gastric cancer is predictive of survival after preoperative therapy[J].Journal of Gastrointestinal Surgery,2018, 22(5):1-9.
[19] Wei-Han Z,Xiao-Hai S,Xin-Zu C,et al.Characteristics and survival outcomes related to the infra-pyloric lymph node status of gastric cancer patients[J]. World Journal of Surgical Oncology,2018,16(1):116.
[20] Kim JW,Lee H,Min YW,et al.Oncologic safety of endoscopic resection based on lymph node metastasis in ulcerative early gastric cancer[J]. Journal of Laparoendoscopic & Advanced Surgical Techniques,2019,29(9):1105-1110.
(收稿日期:2021-02-24)

