纖維素基固態(tài)電解質(zhì)在儲能器件中的應(yīng)用進(jìn)展
張麗榕 王富娟 石小紅 張?zhí)焓|


摘要: 液態(tài)電解質(zhì)具有可燃性,存在起火甚至爆炸等安全隱患,而采用固態(tài)電解質(zhì)代替電解液和隔膜,有望解決安全問題等。纖維素具有環(huán)保價廉、儲量豐富、熱穩(wěn)定性和化學(xué)穩(wěn)定性高、生物相容性好等特點,將其應(yīng)用于儲能器件的固態(tài)電解質(zhì)中,具有降低成本、提高器件工作穩(wěn)定性及使用性能等優(yōu)勢。本文為了全面介紹纖維素基固態(tài)電解質(zhì)在儲能領(lǐng)域的應(yīng)用,首先簡述了纖維素原料在儲能電解質(zhì)應(yīng)用的特征優(yōu)勢;通過分析常見儲能設(shè)備的組成和儲能原理,介紹了固態(tài)電解質(zhì)的性質(zhì)要求;對纖維素基固態(tài)電解質(zhì)在材料和結(jié)構(gòu)設(shè)計方面的研究進(jìn)展進(jìn)行具體說明;并針對電解質(zhì)的結(jié)構(gòu)和性能,論述了常用的表征手段和測試方法;最后指出纖維素基固態(tài)電解質(zhì)的發(fā)展前景,對其存在的問題做了總結(jié)。
關(guān)鍵詞: 天然纖維素;納米纖維素;纖維素結(jié)構(gòu);材料設(shè)計;結(jié)構(gòu)設(shè)計;固態(tài)電解質(zhì);儲能器件
中圖分類號: TS102.1
文獻(xiàn)標(biāo)志碼: A
現(xiàn)代紡織材料中,利用纖維素構(gòu)建能源材料突破了傳統(tǒng)意義上紡織纖維應(yīng)用的領(lǐng)域和概念,已成為可再生資源利用的主要課題之一。近年來,由于電氣化程度的提高與可持續(xù)發(fā)展意識的提升,全球?qū)τ诳稍偕鍧嵞茉吹男枨笈c日俱增。以鋰、鈉電池等為代表的可充電二次電池和超級電容器等儲能器件,因其成本低、效率高、可持續(xù)性好,是目前儲能技術(shù)發(fā)展的重點[1]。電解質(zhì)作為其重要組成,連接著器件的正負(fù)極,同時在器件內(nèi)部傳輸離子,對器件的安全性、穩(wěn)定性和自放電等性能起到至關(guān)重要的作用[2]。
根據(jù)存在狀態(tài),電解質(zhì)可分為液態(tài)電解質(zhì)和固態(tài)電解質(zhì);其中,全固態(tài)電解質(zhì)和準(zhǔn)固態(tài)電解質(zhì)(也稱為凝膠聚合物電解質(zhì))統(tǒng)稱為固態(tài)電解質(zhì)。常見的液態(tài)電解質(zhì)為酸、堿或鹽的水溶液或者有機(jī)電解液體系。由于其較高的離子電導(dǎo)率和對電極極佳的浸潤性,液態(tài)電解質(zhì)在智能手機(jī)、筆記本電腦等便攜式設(shè)備得到廣泛應(yīng)用[3]。然而,液態(tài)電解質(zhì)組裝的儲能設(shè)備其能量密度已接近極限,而且液態(tài)電解質(zhì)有腐蝕性強、易泄露、易燃易爆等缺點,常導(dǎo)致安全事故的發(fā)生,阻礙著應(yīng)用研究的進(jìn)一步發(fā)展[4-5]。為了克服現(xiàn)有商業(yè)液態(tài)儲能器件所面臨的問題,研究者開始大力發(fā)展基于固態(tài)電解質(zhì)的儲能設(shè)備。固態(tài)電解質(zhì)沒有液態(tài)電解質(zhì)體系的上述缺點,使用固態(tài)電解質(zhì)可同時替代大型隔膜和揮發(fā)性電解液[6-7],具有以下優(yōu)勢[8-10]:相對于液態(tài)電解質(zhì),固態(tài)電解質(zhì)不揮發(fā),一般不可燃;一些固態(tài)電解質(zhì)對水分不敏感,在空氣中能保持良好的穩(wěn)定性;具有很寬的電化學(xué)窗口,有利于提高器件的能量密度;固態(tài)電解質(zhì)多為高聚物制備,具有較高的強度和韌性。因此,固態(tài)電解質(zhì)在開發(fā)具有高安全性、高能量密度及功率密度的電動汽車和可穿戴紡織儲能等設(shè)備中發(fā)揮著重要的作用,同時還可協(xié)助這些儲能設(shè)備適用于更寬的溫度范圍[11-13]。
常見的用于固態(tài)電解質(zhì)的基質(zhì)有:聚環(huán)氧乙烷、聚甲基丙烯酸甲酯、聚丙烯腈和纖維素[14-18]等。與其他基質(zhì)相比,纖維素具有來源廣泛、成本低廉和生物相容性好等優(yōu)勢[19],完美契合了儲能技術(shù)綠色、環(huán)保、可持續(xù)的發(fā)展理念,是一種極具發(fā)展前景的儲能材料。此外,以纖維素為基質(zhì)組裝的柔性儲能器件具有優(yōu)異的力學(xué)性能和使用穩(wěn)定性,在經(jīng)受多次循環(huán)外力作用后仍可正常工作。因此,纖維素基固態(tài)電解質(zhì)也常與功能性紡織品結(jié)合,作為驅(qū)動電子皮膚、柔性傳感、能量收集及人體健康監(jiān)測等可穿戴電子設(shè)備的柔性裝置[20]。
本文根據(jù)纖維素的結(jié)構(gòu)和性能,綜合分析了纖維素應(yīng)用于儲能器件中固態(tài)電解質(zhì)領(lǐng)域的潛在優(yōu)勢;從固態(tài)電解質(zhì)的理化性能要求出發(fā),重點討論了纖維素基固態(tài)電解質(zhì)在材料和結(jié)構(gòu)方面的設(shè)計思路;系統(tǒng)介紹了目前纖維素固態(tài)電解質(zhì)的結(jié)構(gòu)和性能表征技術(shù),并進(jìn)一步提出現(xiàn)階段研究的問題和挑戰(zhàn)。
1 纖維素的結(jié)構(gòu)和性能
1.1 纖維素的結(jié)構(gòu)
纖維素主要存在于植物根莖、樹干等木質(zhì)部位的細(xì)胞壁中,同時也存在于細(xì)菌、真菌、藻類甚至是動物細(xì)胞內(nèi)。它是由β-D-吡喃葡萄糖重復(fù)單元組成的線性高分子化合物,化學(xué)式為(C6H10O5)n,其中n表示聚合度,取值范圍從幾百到幾萬。如圖1(a)所示,纖維素的大分子是椅式構(gòu)象[21],每個葡萄糖基環(huán)在2、3、6號碳位上都有游離的羥基[22]。
根據(jù)典型的纓狀原纖理論,纖維素內(nèi)部超微構(gòu)造分結(jié)晶區(qū)和非晶區(qū)兩個基本區(qū)域。非晶區(qū)鏈段上的羥基幾乎為游離態(tài),可通過物理、化學(xué)作用形成具有豐富孔隙的三維網(wǎng)狀結(jié)構(gòu)[23],這樣的結(jié)構(gòu)有利于構(gòu)建可調(diào)節(jié)的孔結(jié)構(gòu),為離子傳輸提供通道,對制備優(yōu)質(zhì)電化學(xué)性能的固態(tài)電解質(zhì)材料起著關(guān)鍵作用。而晶區(qū)鏈段的極性羥基,通過分子內(nèi)與分子間的相互作用形成強結(jié)合力的氫鍵,從而可抵抗外力作用下固態(tài)電解質(zhì)的變形,抑制了枝晶生長。同時,結(jié)晶區(qū)中的所有原子均有固定的位置,這種結(jié)構(gòu)使纖維間結(jié)合得更緊密;而較高的結(jié)晶度使其他分子很難進(jìn)入纖維素的晶體結(jié)構(gòu)中[24]。基于此結(jié)構(gòu)特征,合理設(shè)計纖維素的晶區(qū)和非晶區(qū)的分布比例是開發(fā)其固態(tài)電解質(zhì)應(yīng)用的關(guān)鍵。
1.2 纖維素的性能
纖維素表面含有豐富的羥基,具有良好的親水性[25],對提高電解液的潤濕性有積極作用。也正是因為大分子上的這些羥基與其他極性基團(tuán)的存在,使得纖維素分子間和分子內(nèi)部作用力變強。通過氧化作用獲得的表面羧基可促進(jìn)離子的遷移,提高其離子導(dǎo)電性,并且協(xié)同提高了電解質(zhì)的強度和韌性[26],對于柔性儲能設(shè)備的設(shè)計具有極大吸引力。此外,纖維素具有較好的熱穩(wěn)定性,熱解溫度達(dá)到250 ℃,可避免電池因長期或高溫工作引起的安全問題。根據(jù)這一性質(zhì),已有研究者制備了纖維素基的阻燃及耐高溫型電解質(zhì)[27]。纖維素基固態(tài)電解質(zhì)在不同儲能器件中應(yīng)用的性能優(yōu)勢如表1所示。由表1可看出,與其他各類聚合物固態(tài)電解質(zhì)、無機(jī)固態(tài)電解質(zhì)、凝膠電解質(zhì)等比較,纖維素基固態(tài)電解質(zhì)在離子電導(dǎo)率、離子轉(zhuǎn)移數(shù)、力學(xué)性能和器件電容保持率等方面均有優(yōu)勢。
近年來,納米纖維素因強度高、質(zhì)量輕、尺寸納米化和比表面積大等特點,在固態(tài)電解質(zhì)領(lǐng)域被廣泛應(yīng)用。如圖1(b)[28]所示,根據(jù)來源、制備方法和纖維形態(tài)的不同,納米纖維素可分為以下三類[29]:1) 纖維素納米纖維(Cellulose Nanofiber,CNF)。CNF是在高壓或機(jī)械作用下得到的納米纖維素纖維,具有大長徑比,交聯(lián)點多的優(yōu)勢。即使在非水介質(zhì)中CNF也容易形成3D網(wǎng)絡(luò)結(jié)構(gòu)。另外由于CNF質(zhì)量輕,其楊氏模量與高強度的芳綸相當(dāng);熱膨脹性質(zhì)又與玻璃相似,因此它在制備輕質(zhì)、高性能固態(tài)電解質(zhì)方面具有很大的潛力[30]。2) 纖維素納米晶(Cellulose Nanocrystals,CNC)。它是在酸水解下形成的具有高結(jié)晶度的納米級棒狀顆粒,長徑比小,具有形成分層介孔結(jié)構(gòu)的能力。所以可促進(jìn)離子轉(zhuǎn)移進(jìn)而均勻電極間的離子通量,為電池提供穩(wěn)定和可逆的離子電鍍剝離。3) 細(xì)菌纖維素(Bacterial Nanocellulose,BC)。BC是指在微生物發(fā)酵得到的結(jié)晶度在95%以上的納米纖維。表面含大量氫鍵,可形成豐富孔隙的超細(xì)網(wǎng)絡(luò)結(jié)構(gòu),吸水性和機(jī)械強度高,具有良好的理化穩(wěn)定性[31-32]。
2 固態(tài)電解質(zhì)的理化性質(zhì)要求
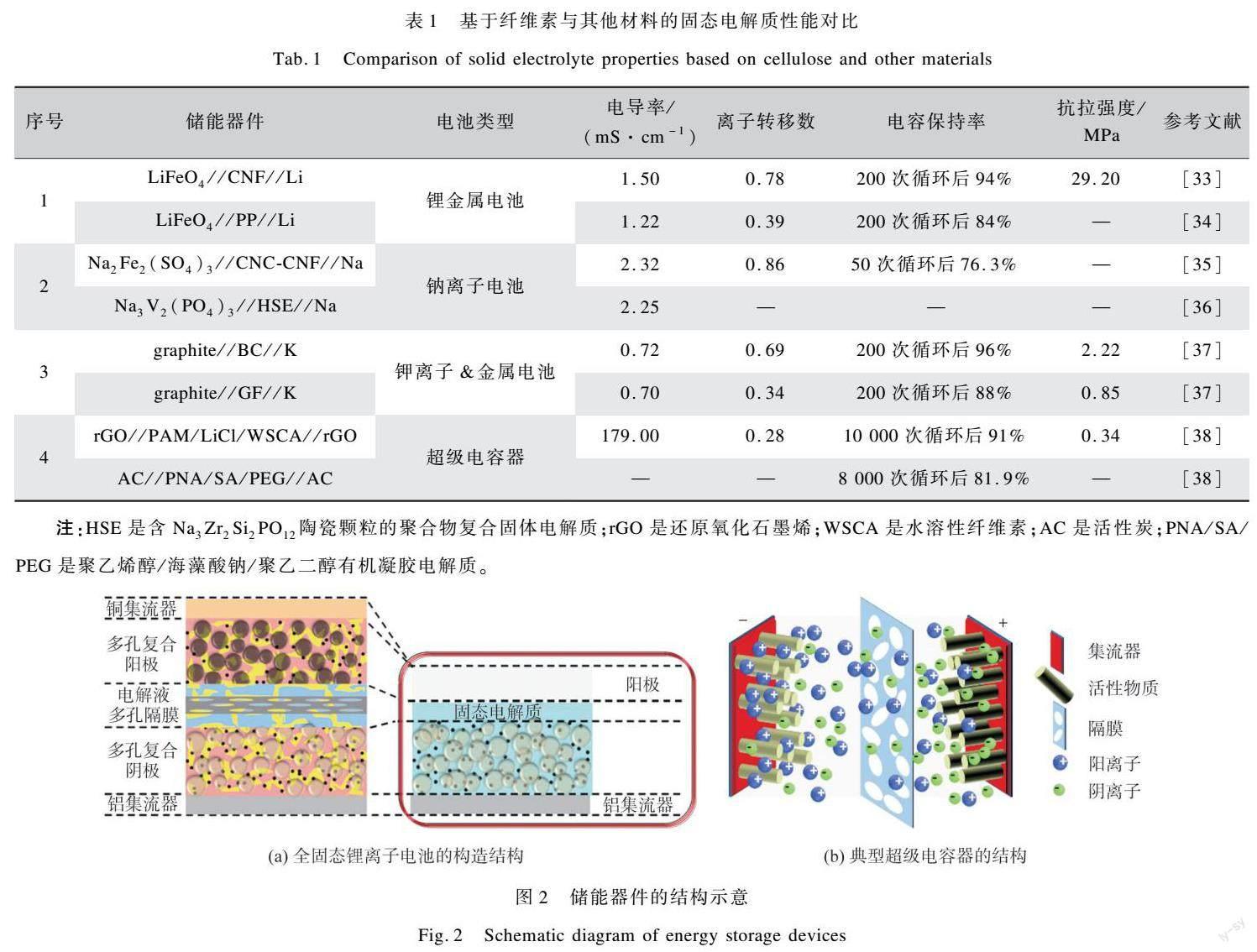
現(xiàn)在研究的主流儲能器件包括鋰、鈉、鉀、鋅等技術(shù)成熟的可充電二次電池和超級電容器。傳統(tǒng)的二次電池由正負(fù)極、隔膜和電解液等組成。固態(tài)電池是由正負(fù)極、固態(tài)電解質(zhì)等組成。它通過自發(fā)的電極反應(yīng)實現(xiàn)化學(xué)能到電能的轉(zhuǎn)換[39],充電時,離子從正極流向負(fù)極進(jìn)行還原反應(yīng),放電過程與充電過程相反。以鋰電為例,其器件組裝和工作原理如圖2(a)[40]所示。與化學(xué)電源相比,超級電容器的儲能機(jī)理與電池的完全不同,但是其結(jié)構(gòu)組成與電池類似[41],如圖2(b)所示。超電的儲能過程高度可逆,可提供高于傳統(tǒng)電容器幾個數(shù)量級的能量密度,同時有著壽命長和充放電速率高的特點[42]。根據(jù)工作原理,超級電容器分為雙電層電容器和贗電容電容器。其中雙電層超級電容器通過離子在電極表面的吸附和解吸附電解液的離子來實現(xiàn)儲存能量;贗電容型超級電容器主要通過電極表面的氧化還原完成儲能[43]。除此之外,還有電池型電容器是指將電池材料與超級電容器材料相結(jié)合,通過電極材料體相和表面的可逆法拉第反應(yīng)完成電荷的積累[44]。
為協(xié)助其他組件實現(xiàn)儲能器件的高性能和優(yōu)異的穩(wěn)定性,固態(tài)電解質(zhì)需滿足以下基本要求:1) 高離子電導(dǎo)率。離子電導(dǎo)率高有利于離子的運輸,并有限制器件本身自放電的作用[45-46]。當(dāng)器件內(nèi)部電阻降低時,可減少其運行過熱而產(chǎn)生的安全問題。2) 良好的界面接觸。固態(tài)電解質(zhì)無法像液態(tài)電解質(zhì)那樣具有良好的浸潤性,與電極之間的接觸面積較小,存在電極/電解質(zhì)多級界面反應(yīng)和電子/離子傳輸問題。良好的界面接觸可有效抑制界面阻抗的增加,有利于界面電荷的傳輸[47-49]。3) 寬電化學(xué)窗口。為保持器件內(nèi)部氧化還原反應(yīng)的可逆性和穩(wěn)定性,避免副反應(yīng)的產(chǎn)生,氧化還原反應(yīng)的電勢差即電化學(xué)窗口應(yīng)足夠?qū)挕k娀瘜W(xué)窗口寬,電解質(zhì)不易在充放電過程中分解,也有利于電極與電解質(zhì)的界面穩(wěn)定[50-51]。4) 機(jī)械強度高。良好的機(jī)械強度可抑制金屬負(fù)極枝晶的生長,這也是固態(tài)電解質(zhì)可以匹配金屬負(fù)極的原因之一[52-53]。5) 環(huán)境穩(wěn)定性高。水系儲能器件在組裝過程中,固態(tài)電解質(zhì)不可避免暴露于空氣中,其化學(xué)性質(zhì)和結(jié)構(gòu)可能會發(fā)生變化,導(dǎo)致其離子運輸特性和機(jī)械完整性改變。因此,具有較好的環(huán)境穩(wěn)定性的固態(tài)電解質(zhì)對保證固態(tài)儲能器件的高效、安全運行非常重要[54-55]。
與液態(tài)電解質(zhì)相比,固態(tài)電解質(zhì)可在一定程度上緩解電化學(xué)引起的應(yīng)變,但它的應(yīng)力應(yīng)變傳遞使得電化學(xué)性能與力學(xué)性能的耦合問題更加嚴(yán)峻。因此,在設(shè)計高性能固態(tài)電解質(zhì)時,要綜合考量電化學(xué)性能與力學(xué)穩(wěn)定性之間的關(guān)系。根據(jù)纖維素結(jié)構(gòu)和性能特點可知,纖維素具有開發(fā)成為高導(dǎo)電率、良好界面穩(wěn)定性、機(jī)械性能和環(huán)境穩(wěn)定性的固態(tài)電解質(zhì)的潛力和優(yōu)勢,可將其用于儲能器件的開發(fā)中。
3 纖維素基固態(tài)電解質(zhì)的設(shè)計
3.1 纖維素基固態(tài)電解質(zhì)的材料設(shè)計
電解質(zhì)材料是電解質(zhì)性能設(shè)計的基礎(chǔ),對應(yīng)不同性質(zhì)要求來選擇合適的原料是制備纖維素基固態(tài)電解質(zhì)的關(guān)鍵。固態(tài)電解質(zhì)常用的材料設(shè)計主要有兩個途徑:纖維素自身改性,纖維素與其他材料的復(fù)合。
未經(jīng)改性的纖維素內(nèi)部氫鍵作用力強,無定形結(jié)構(gòu)少,這不利于離子在纖維素內(nèi)部傳輸。故纖維素作為電解質(zhì)基材的使用,首先應(yīng)改變的是纖維素材料內(nèi)部的強氫鍵網(wǎng)絡(luò),可采用化學(xué)改性方法優(yōu)化纖維素的表面性質(zhì)或纖維素的內(nèi)部結(jié)構(gòu)。Ge等[56]用磺酸鹽對CNF進(jìn)行功能化改性,將離子轉(zhuǎn)移數(shù)從0.12提高到了0.70左右,同時改善了電解質(zhì)的韌性、親水性及均勻了孔隙分布,為實用型電解質(zhì)的設(shè)計提出了一個新方向。在調(diào)節(jié)鋰離子電池的離子運輸、維持電極的穩(wěn)定性及提高電池的安全性設(shè)計的過程中,Zhu等[57]用1-丁基-3-甲基咪唑氯鹽([Bmim]Cl)離子液體處理纖維素,打破了纖維素內(nèi)部分子結(jié)構(gòu)的氫鍵,并將其制成漿液。隨后,纖維素漿液緩慢有序地滲入玻璃纖維的三維網(wǎng)絡(luò)結(jié)構(gòu)中。因纖維素大分子的氫鍵作用和玻璃纖維的空間位阻作用,液態(tài)纖維素轉(zhuǎn)化為固相之后,即可形成具有梯度層次變化的多孔隔膜,如圖3(a)所示。可調(diào)節(jié)的梯度孔隙結(jié)構(gòu)有利于離子的快速傳輸,使電池具有良好的循環(huán)穩(wěn)定性,在循環(huán)1 000次后其比容量仍可保持到108.5 mAh/g。
眾多纖維素的化學(xué)組成雖然一致,相互之間親和性也較好,但其物理性質(zhì)差異明顯。將不同的纖維素復(fù)合,可取長補短、剛?cè)岵?jì),進(jìn)一步發(fā)揮纖維素材料的結(jié)構(gòu)和性能優(yōu)勢。Mittal等[35]將這一理念應(yīng)用在鈉離子電池,為可持續(xù)能源儲存系統(tǒng)提供了新的策略,通過戊二醛將剛性較強的CNC和柔性較好的CNF交聯(lián),獲得具有豐富孔隙結(jié)構(gòu)的凝膠電解質(zhì)(GPE),如圖3(b)所示。CNC上的—OH、—OSO-3與CNF上的—COO-、—OH協(xié)同提高了Na+移動能力,使得Na+能在有機(jī)液體電解質(zhì)存在的情況下快速通過GPE。該GPE將離子電導(dǎo)率和離子轉(zhuǎn)移數(shù)分別提高到了2.32 mS/cm和0.637,與Na2Fe2(SO4)3正極和Na負(fù)極組裝,電池的能量密度達(dá)到了240 Wh/kg。與商用隔膜相比,GPE在離子電導(dǎo)率、離子遷移數(shù)和電化學(xué)穩(wěn)定性之間取得了很好的平衡。
此外,部分無機(jī)和高分子材料已在電化學(xué)領(lǐng)域有較好的表現(xiàn),將其與密度小、力學(xué)性能好、環(huán)境穩(wěn)定性強的纖維素材料復(fù)合,可進(jìn)一步優(yōu)化、提升儲能器件的能量密度和使用性能。Zhu等[58]提出了一種纖維素協(xié)助法,制備出了一種超薄(60 μm)的柔性硫銀鍺礦型(Li6PS5Cl)電解質(zhì)薄膜,大幅降低了非活性層的電阻和質(zhì)量,從而提高了器件的能量密度。同時,纖維素與無機(jī)顆粒間有很強的作用,膜的厚度不會隨Li6PS5Cl懸浮液澆筑而增加。因此,纖維素的引入不僅降低了固態(tài)電解質(zhì)的厚度,還改善了固態(tài)電解質(zhì)膜的力學(xué)性能,而且組裝的鋰金屬電池工作循環(huán)過程中,電解質(zhì)內(nèi)部形成穩(wěn)定的離子傳輸通道,其離子電導(dǎo)率可達(dá)6.3 mS/cm,實現(xiàn)了鋰離子的快速運輸。Zhang等[8]將丙烯酰胺單體在CNF上原位聚合,制備具有良好保水性質(zhì)的聚丙烯酰胺(PAM)-CNF堿性雙網(wǎng)絡(luò)水凝膠電解質(zhì),如圖3(c)所示。其中,CNF與PAM之間的物理纏結(jié)和非共價作用顯著改善了電解質(zhì)的力學(xué)性能,調(diào)節(jié)了納米孔結(jié)構(gòu),有效提高了離子的傳輸能力。由于纖維素的加入,電解質(zhì)的抗脫水和抗凍性能提升,該電池可以在-40 ℃環(huán)境下工作,有望與其他電子設(shè)備結(jié)合實現(xiàn)低溫型可穿戴儲能設(shè)備的開發(fā)。纖維素基固態(tài)電解質(zhì)在超級電容器中也被廣泛應(yīng)用。Zhang等[38]合成了高溶解度的水溶性醋酸纖維素(WSCA),與PAM和LiCl共同制備出凝膠電解質(zhì)。由于氫鍵和PAM/LiCl/WSCA基質(zhì)中的協(xié)同作用,該電解質(zhì)具有極佳的力學(xué)性質(zhì),其應(yīng)力為341 kPa,韌性達(dá)1.2 MJ/m3。將其組裝成超級電容器后,經(jīng)過10 000次充放電循環(huán)測試發(fā)現(xiàn),電容保持率高達(dá)91%,并且在-40 ℃仍能保持64.64%的容量和良好的柔性,為低溫環(huán)境下的電解質(zhì)應(yīng)用提供了新途徑。
3.2 纖維素基固態(tài)電解質(zhì)的結(jié)構(gòu)設(shè)計
纖維素的結(jié)構(gòu)有形態(tài)結(jié)構(gòu)、聚集態(tài)結(jié)構(gòu)及更微觀的分子結(jié)構(gòu)。在纖維素不同層次進(jìn)行結(jié)構(gòu)設(shè)計,通過優(yōu)化纖維素的內(nèi)部孔隙和結(jié)晶結(jié)構(gòu),從而改善非活性物質(zhì)的團(tuán)聚現(xiàn)象、增加離子傳輸?shù)穆窂健⑻岣邆鬏斁W(wǎng)絡(luò)的有序度,為固態(tài)電解質(zhì)的發(fā)展注入新的活力。
纖維素的形態(tài)結(jié)構(gòu)設(shè)計包括對其外觀形貌、表面結(jié)構(gòu)、截面結(jié)構(gòu)及各種裂隙和孔洞的設(shè)計,其中對孔的設(shè)計是重點。Xie等[59]使BC吸收前驅(qū)鹽溶液后,將其煅燒生成多孔的立方相石榴石型納米纖維網(wǎng)絡(luò)。進(jìn)一步,制備成BC基固態(tài)電解質(zhì),縮短了離子的運輸途徑,電導(dǎo)率可達(dá)到1.12×10-4 S/cm。此外,這種電解質(zhì)不僅可以對電池工作過程中產(chǎn)生的應(yīng)力起到緩沖作用,還可改善電極和電解質(zhì)之間的界面問題,顯著抑制在循環(huán)過程中不均勻的鋰沉積。Zhu等[60]用非溶劑蒸發(fā)法設(shè)計了一種多孔隙的凝膠電解質(zhì)膜,即在CMC懸浮液中加入沸點不同的蒸餾水和N,N-二甲基甲酰基(DMF)。通過改變DMF和水的比例,調(diào)節(jié)凝膠電解質(zhì)膜的孔隙結(jié)構(gòu)。用1 mol/L的LiPF6浸泡后,離子電導(dǎo)率提高到0.48 mS/cm,與室溫下的商用隔膜Celgard 2730相比,離子遷移率要高出一倍。
改變纖維素大分子鏈堆砌方式是對聚集態(tài)結(jié)構(gòu)設(shè)計的主要方式,即調(diào)整纖維素結(jié)晶、非晶結(jié)構(gòu)的比例,在保證其力學(xué)性能的同時使離子有足夠的通道傳輸、擴(kuò)散。如圖4(a)所示,Yang等[33]利用Cu2+與CNF配位,通過擴(kuò)大聚合物鏈之間的間距,使分子通道能夠插入和快速運輸Li+,從而改變纖維素的晶體結(jié)構(gòu)。在這個一維傳輸通道中,纖維素中的豐富官能團(tuán)、少量的結(jié)合水,與聚合物節(jié)段解耦,從而達(dá)到了協(xié)助Li+運動的目的。因此,制備的Li-Cu-CNF離子導(dǎo)體將電化學(xué)窗口拓寬到4.5 V,同時具有高離子電導(dǎo)率(1.5×10-3 S/cm)和高離子遷移數(shù)(0.78),如圖4(b)所示。這種方法為制備高能量密度和高安全性的電池提供了一種可靠的思路。
根據(jù)儲能用固態(tài)電解質(zhì)的性能要求,對纖維素更小分子層次進(jìn)行結(jié)構(gòu)設(shè)計,通過改善內(nèi)部官能團(tuán),引入化學(xué)鍵作用等方式,進(jìn)一步提升纖維素基固態(tài)電解質(zhì)的性能。Ma等[61]制備同時具有共價交聯(lián)和氫鍵作用的聚丙烯酸鈉(PANa)纖維素雙網(wǎng)絡(luò)水凝膠。通過PANa在纖維素鏈上的反應(yīng)增強了水凝膠電解質(zhì)的機(jī)械強度和拉伸性能,組裝的鋅空氣電池的功率密度為108.6 mW/cm2,拉伸800%后功率密度增加到210.5 mW/cm2。Wang等[62]用2-溴丙酰溴對纖維素微晶進(jìn)行表面化學(xué)改性,通過原子轉(zhuǎn)移自由基聚合法將離子導(dǎo)電聚合物段可控的共價接枝在纖維素上。如圖4(c)(d)所示,制備的纖維素基全固態(tài)聚合物電解質(zhì)(CSSPE)中具有豐富的離子通道,其離子電導(dǎo)率達(dá)到8×10-5 S/cm。此外,該電解質(zhì)還將電池的電化學(xué)窗口拓寬到了4.9 V,具備抑制枝晶生長的能力,組裝的CSSPE全電池循環(huán)450次后庫倫效率超過99%。
4 纖維素基固態(tài)電解質(zhì)的表征技術(shù)
電解質(zhì)的結(jié)構(gòu)決定著其物理化學(xué)穩(wěn)定性、內(nèi)部離子的電化學(xué)遷移能力及與電極的相容程度。電池工作過程中,離子傳導(dǎo)又涉及多相介質(zhì)和異相界面[63],因此對電解質(zhì)的性質(zhì)有較高的要求。準(zhǔn)確地表征和測試有助于了解纖維素基固體電解質(zhì)的性能,可有針對性地指導(dǎo)固態(tài)器件的開發(fā)與設(shè)計。
4.1 結(jié)構(gòu)表征
結(jié)構(gòu)表征是性質(zhì)分析的基礎(chǔ)。固態(tài)電解質(zhì)的離子傳輸對電解質(zhì)的孔隙、缺陷、空位分布的依賴性較強,所以其形貌、官能團(tuán)變化、內(nèi)部晶體等結(jié)構(gòu)的準(zhǔn)確呈現(xiàn)有助于后續(xù)結(jié)構(gòu)的穩(wěn)定性及電化學(xué)性質(zhì)進(jìn)行協(xié)助分析。固態(tài)電解質(zhì)常用的結(jié)構(gòu)表征手段有:直觀獲取表面微觀形貌結(jié)構(gòu)、孔徑分布和尺寸等物理和化學(xué)信息的掃描電子顯微鏡(SEM);通過測試不同波長紅外光的吸收峰對應(yīng)的頻率,分析電解質(zhì)的分子基團(tuán)、化學(xué)鍵類別與大致相對含量,以及化學(xué)環(huán)境變化的傅里葉變換紅外光譜(FT-IR);解析光譜中的分子化學(xué)鍵和基團(tuán)進(jìn)而對分子結(jié)構(gòu)進(jìn)行推測的拉曼光譜;利用X射線衍射儀檢測電解質(zhì)結(jié)晶信息和物象組成的XRD,分析電解質(zhì)元素和原子價態(tài)構(gòu)成的XPS。Lei等[64]制備了高離子電導(dǎo)率的細(xì)菌纖維素基凝膠聚合物電解質(zhì)(BC-GPES)。BC-GPES表面和裂縫處的SEM圖像如圖5(a)(b)所示。當(dāng)在BC-GPES中加入常規(guī)有機(jī)電解液一段時間后,得到BC-GPE。如圖5(c)所示,電解液均勻填充在支架的孔隙中。說明電解液與BC-GPES之間的相互作用,促進(jìn)了電解質(zhì)凝膠化的形成。如圖5(d)所示,觀察浸潤電解液前后的FTIR曲線,BC-GPES曲線上的CO峰表明,在凝膠化過程中電解液進(jìn)入BC-GPES,從而提高凝膠電解質(zhì)的電導(dǎo)率。
固態(tài)電解質(zhì)與電解液的親疏性直接影響著離子的電導(dǎo)率、電極與電解質(zhì)之間的界面問題。電解質(zhì)與電解液的親疏性可用電解液的吸收率和保留率來表示,具體方法是對比浸泡或蒸發(fā)電解液前后電解質(zhì)的質(zhì)量變化。如Zhu等[57]將隔膜浸泡電解液后測試其吸收率超過550%,保留率達(dá)到73%。同時,電解質(zhì)材料的比表面積、孔容和孔徑分布等與電化學(xué)性能也密切相關(guān)。氮氣吸附法是目前最常用的方法,如圖5(e)所示。當(dāng)凝膠的比表面積較大時,有利于離子的通過,從而提高離子導(dǎo)電率。此外,均勻的孔徑可確保電極間足夠的Na+通量,有促進(jìn)Na+均勻沉積的作用[35]。纖維素基電解質(zhì)內(nèi)部的非晶區(qū)域的比例,可用孔隙率和葡萄糖單元(AGU)堆積密度來表示。AGU堆積密度反映了Li-Cu-CNF的非晶區(qū)占比,即堆積密度越大,非晶區(qū)占比變小,從而離子通道空間越小[33]。當(dāng)Cu︰AGU=1︰12時,通道完全打開,如圖5(f)所示。
4.2 理化性質(zhì)表征
電池在裝配和使用過程中會受到外力的擠壓、彎曲和沖撞作用,因此測試固態(tài)電解質(zhì)的力學(xué)性質(zhì)非常有必要。通過重復(fù)循環(huán)拉伸、折疊、卷曲、擠壓和扭轉(zhuǎn)試驗,分析電解質(zhì)的剛性、韌性、彈性及力學(xué)穩(wěn)定性等信息,為固態(tài)電解質(zhì)在今后的組裝和使用打下堅實的基礎(chǔ)[8]。為驗證BC的加入對PEO/LiTFSI復(fù)合固態(tài)電解質(zhì)(CSPE)力學(xué)性質(zhì)的影響,對BC加入前后制備的固態(tài)電解質(zhì)做了拉伸測試[65],如圖6(a)所示。由應(yīng)力應(yīng)變曲線可看出,BC加入對復(fù)合固態(tài)電解質(zhì)拉伸強度的提高有很大的幫助。同時,力學(xué)性質(zhì)也是衡量電解質(zhì)抑制枝晶生長能力的指標(biāo)之一,影響著器件的長循環(huán)性能。Wang等[37]制備的分層多孔纖維素電解質(zhì)隔膜,由于BC的加入將抗拉強度提高到2.22 MPa,達(dá)到了抑制枝晶的目的,對應(yīng)的對稱電池循環(huán)壽命超過了1 000 h。
電解質(zhì)的熱穩(wěn)定性關(guān)系到器件的應(yīng)用安全性,可由熱重分析(TGA)和差示掃描熱分析(DSC)曲線獲得固態(tài)電解質(zhì)的分解溫度、結(jié)晶程度、氧化反應(yīng)過程等熱力學(xué)和反應(yīng)動力學(xué)參數(shù)。Wang等[62]將離子導(dǎo)電段聚合到纖維素上形成刷狀結(jié)構(gòu),制備的纖維素基固態(tài)電解質(zhì)離子通道得以擴(kuò)充。聚合物鏈連接到纖維素前后的熱分解溫度沒有改變,保持在305 ℃,如圖6(b)所示。另與纖維素聚合物的DSC曲線相比,纖維素基電解質(zhì)的熔融峰完全消失,玻璃化轉(zhuǎn)變溫度為38 ℃,如圖6(c)所示。說明鋰鹽與聚合物基體有較強的相互作用鏈段,有利于纖維素基全固態(tài)聚合物電解質(zhì)熱穩(wěn)定性的提高。
4.3 電化學(xué)性能表征
電化學(xué)測試是表征電解質(zhì)性能中最為核心關(guān)鍵的步驟,其電化學(xué)性能的測試與器件、電極的測試方法類似,主要包括線性掃描伏安測試、交流阻抗測試和恒流充放電測試。但獲取的參數(shù)有所區(qū)別,包括電化學(xué)窗口、離子電導(dǎo)率和循環(huán)性能的指標(biāo)(循環(huán)次數(shù)及容量保持率)等。電化學(xué)穩(wěn)定窗口即電解質(zhì)能正常工作的電壓范圍,是用來衡量器件能否商業(yè)化的重要指標(biāo)之一。在電化學(xué)工作站設(shè)定掃描范圍與掃速大小,通過線性掃描伏安法測得對應(yīng)氧化分解電壓,常用CV曲線表征。Yang等[33]制備Cu2+配位的Li-Cu-CNF纖維素離子導(dǎo)體,在掃速0.1 mV/s、電流密度≤10-6 A/cm2下表現(xiàn)出0~4.5 V的寬電化學(xué)窗口,這將有助于能量密度的提升,如圖6(d)所示。離子電導(dǎo)率σ(S/cm)是表征離子導(dǎo)電性最重要參數(shù)之一,一般由電化學(xué)工作站通過阻塞電極法獲得,即通過電化學(xué)交流阻抗測試,得到電解質(zhì)的本體阻抗Rb(Ω),如圖6(e)所示。由公式σ=d/(Rb×A),其中d為電解質(zhì)厚度(cm)、A為阻塞電極與電解質(zhì)膜的接觸面積(cm2),計算得出電解質(zhì)的離子電導(dǎo)率是1.5×10-3 S/cm。而循環(huán)穩(wěn)定性能通常采用藍(lán)電測試系統(tǒng)通過恒流充放電測試獲得,以此來評估電解質(zhì)在儲能器件中的實際應(yīng)用能力。Cui等[56]開發(fā)了一種基于單離子功能化的CNF多功能隔膜,用其組裝的鋅金屬電池在1 mA/cm2的電流密度下,在0.5 mAh/cm2面積容量時有超過500圈的超長循環(huán)壽命,并具有穩(wěn)定的極化作用,如圖6(f)所示。
5 結(jié) 論
纖維素基固態(tài)電解質(zhì)的設(shè)計與開發(fā)是目前實現(xiàn)綠色高效儲能的重要橋梁。本文結(jié)合對固態(tài)電解質(zhì)的性能要求,對纖維素的結(jié)構(gòu)與性能做出簡要分析,對其作為固態(tài)電解質(zhì)在常見儲能器件的應(yīng)用進(jìn)行了綜述。得出以下結(jié)論:1) 對纖維素進(jìn)行功能化改性和結(jié)構(gòu)設(shè)計,得到的纖維素基固態(tài)電解質(zhì)對儲能器件的電化學(xué)性能及穩(wěn)定性有提升作用。2) 針對纖維素分子鏈的結(jié)構(gòu)設(shè)計,可擴(kuò)大纖維素在固態(tài)電解質(zhì)領(lǐng)域的應(yīng)用前景。3) 對于纖維素基固態(tài)電解質(zhì)的結(jié)構(gòu)設(shè)計,孔的合理設(shè)計是關(guān)鍵,而孔徑大小和孔徑分布是研究的重點。4) 對纖維素基固態(tài)電解質(zhì)進(jìn)行結(jié)構(gòu)、理化和電化學(xué)性質(zhì)的測試表征,得到的電解質(zhì)基本性質(zhì)可對今后纖維素基固態(tài)電解質(zhì)的設(shè)計、制備進(jìn)行指導(dǎo)。
未來纖維素基固態(tài)電解質(zhì)仍有以下問題需要解決:1) 纖維素基固態(tài)電解質(zhì)的制備過程繁瑣、耗時耗能,需優(yōu)化制備工藝和尋找綠色助劑。2) 性能還需繼續(xù)完善,如離子遷移數(shù)、與電極的界面相容性、力學(xué)性能等均需進(jìn)一步提升。3) 大多數(shù)電解質(zhì)的研究與制備都局限在實驗室水平,并未實現(xiàn)由實驗室到工廠的產(chǎn)業(yè)化轉(zhuǎn)變。4) 目前纖維素大多還只是作為固態(tài)電解質(zhì)的材料基質(zhì)使用,而對纖維素本身的聚集態(tài)結(jié)構(gòu)和微觀分子結(jié)構(gòu)設(shè)計較少。
為繼續(xù)推動纖維素固態(tài)電解質(zhì)在儲能領(lǐng)域中的應(yīng)用,需在不損失纖維素機(jī)械性能的前提下,繼續(xù)優(yōu)化纖維素基固態(tài)電解質(zhì)的離子導(dǎo)電性和化學(xué)穩(wěn)定性,使其在可穿戴式柔性電子設(shè)備上得到廣泛的應(yīng)用。
《絲綢》官網(wǎng)下載
中國知網(wǎng)下載
參考文獻(xiàn):
[1]FAESSLER B. Stationary, second use battery energy storage systems and their applications: A research review[J]. Energies, 2021, 14(8): 1-19.
[2]LI W, PANG Y, LIU J, et al. A PEO-based gel polymer electrolyte for lithium ion batteries[J]. RSC Advances, 2017, 7(38): 23494-23501.
[3]MANTHIRAM A. A reflection on lithium-ion battery cathode chemistry[J]. Nature Communications, 2020, 11(1): 1-9.
[4]CANO Z P, BANHAM D, YE S, et al. Batteries and fuel cells for emerging electric vehicle markets[J]. Nature Energy, 2018, 3(4): 279-289.
[5]宋鑫, 高志浩, 駱林, 等. 全固態(tài)鋰電池有機(jī)無機(jī)復(fù)合電解質(zhì)研究進(jìn)展[J]. 復(fù)合材料學(xué)報, 2022, 40: 1-18.
SONG Xin, GAO Zhihao, LUO Lin, et al. Research progress of organic-inorganic composite electrolytes for allsolid-state lithium batteries[J]. Journal of Composite Materials, 2022, 40: 1-18.
[6]FENECH M, SHARMA N. Pulsed laser deposition-based thin film microbatteries[J]. Chemistry, 2020, 15(12): 1829-1847.
[7]LIU M, ZHANG S, VAN ECK E R H, et al. Improving Li-ion interfacial transport in hybrid solid electrolytes[J]. Nature Nanotechnology, 2022, 17(9): 1-19.
[8]ZHANG Y, QIN H, ALFRED M, et al. Reaction modifier system enable double-network hydrogel electrolyte for flexible zinc-air batteries with tolerance to extreme cold conditions[J]. Energy Storage Materials, 2021, 42: 88-96.
[9]CHEN R, LI Q, YU X, et al. Approaching practically accessible solid-state batteries: Stability issues related to solid electrolytes and interfaces[J]. Chemical Reviews, 2020, 120(14): 6820-6877.
[10]XIA S, YANG B, ZHANG H, et al. Ultrathin layered double hydroxide nanosheets enabling composite polymer electrolyte for all-solid-state lithium batteries at room temperature[J]. Advanced Functional Materials, 2021, 31(28): 1-11.
[11]ZHENG Y, YAO Y, OU J, et al. A review of composite solid-state electrolytes for lithium batteries: Fundamentals, key materials and advanced structures[J]. Chemical Society Reviews, 2020, 49(23): 8790-8839.
[12]MANTHIRAM A, YU X, WANG S. Lithium battery chemistries enabled by solid-state electrolytes[J]. Nature Reviews Materials, 2017, 2(4): 1-16.
[13]WAN J, XIE J, MACKANIC D G, et al. Status, promises, and challenges of nanocomposite solid-state electrolytes for safe and high performance lithium batteries[J]. Materials Today Nano, 2018, 4: 1-16.
[14]MAKHLOOGHIAZAD F, ODELL L A, PORCARELLI L, et al.
Zwitterionic materials with disorder and plasticity and their application as non-volatile solid or liquid electrolytes[J]. Nature Materials, 2022, 21(2): 228-236.
[15]HE W, CUI Z, LIU X, et al. Carbonate-linked poly(ethylene oxide) polymer electrolytes towards high performance solid state lithium batteries[J]. Electrochimica Acta, 2017, 225: 151-159.
[16]XUE Z, HE D, XIE X. Poly (ethylene oxide)-based electrolytes for lithium-ion batteries[J]. Journal of Materials Chemistry A, 2015, 3(38): 19218-19253.
[17]HE M, ZHANG X, JIANG K, et al. Pure inorganic separator for lithium ion batteries[J]. ACS Applied Materials & Interfaces, 2015, 7(1): 738-742.
[18]趙德方, 鄧安國, 黃芽, 等. 聚丙烯超細(xì)短纖維鋰離子電池隔膜的制備及性能研究[J]. 絲綢, 2022, 59(4): 24-30.
ZHAO Defang, DENG Anguo, HUANG Ya, et al. Study on the preparation and properties of superfine short polypropylene lithium-ion battery separators[J]. Journal of Silk, 2022, 59(4): 24-30.
[19]RAHMANIAN V, PIRZADA T, WANG S, et al. Cellulose-based hybrid aerogels: Strategies toward design and functionality[J]. Advanced Materials, 2021, 33(51): 1-26.
[20]姜志潔. 納米纖維素基超級電容器的研究進(jìn)展[J]. 合成化學(xué), 2022, 30(6): 510-518.
JIANG Zhijie. Research progress of nanocellulose-based supercapacitors[J]. Chinese Journal of Synthetic Chemistry, 2022, 30(6): 510-518.
[21]MOON R J, MARTINI A, NAIRN J, et al. Cellulose nanomaterials review: Structure, properties and nanocomposites[J]. Chemical Society Reviews, 2011, 40(7): 3941-3994.
[22]DE SOUZA LIMA M M, BORSALI R. Rodlike cellulose microcrystals: Structure, properties, and applications[J]. Macromolecular Rapid Communications, 2004, 25(7): 771-787.
[23]PAN R, CHEUNG O, WANG Z, et al. Mesoporous cladophora cellulose separators for lithium-ion batteries[J]. Journal of Power Sources, 2016, 321: 185-192.
[24]CAZN P, VZQUEZ M. Improving bacterial cellulose films by ex-situ and in-situ modifications: A review[J]. Food Hydrocolloids, 2021, 113: 1-9.
[25]OLSSON R T, AZIZI SAMIR M A S, SALAZAR-ALVAREZ G, et al. Making flexible magnetic aerogels and stiff magnetic nanopaper using cellulose nanofibrils as templates[J]. Nature Nanotechnology, 2010, 5(8): 584-588.
[26]YE Y, ZHANG Y, CHEN Y, et al. Cellulose nanofibrils enhanced, strong, stretchable, freezing-tolerant ionic conductive organohydrogel for multi-functional sensors[J]. Advanced Functional Materials, 2020, 30(35): 1-12.
[27]YANG J, ZHANG M, CHEN Z, et al. Flame-retardant quasi-solid polymer electrolyte enabling sodium metal batteries with highly safe characteristic and superior cycling stability[J]. Nano Research, 2019, 12(9): 2230-2237.
[28]XU T, DU H, LIU H, et al. Advanced nanocellulose-based composites for flexible functional energy storage devices[J]. Advanced Materials, 2021, 33(48): 1-30.
[29]GONZALEZ-GIL R M, BORRAS M, CHBANI A, et al. Sustainable and printable nanocellulose-based ionogels as gel polymer electrolytes for supercapacitors[J]. Nanomaterials (Basel), 2022, 12(2): 1-14.
[30]LI J, DAI L, WANG Z, et al. Cellulose nanofiber separator for suppressing shuttle effect and Li dendrite formation in lithium-sulfur batteries[J]. Journal of Energy Chemistry, 2022, 67: 736-744.
[31]ZHANG T, YANG L, YAN X, et al. Recent advances of cellulose-based materials and their promising application in sodiumion batteries and capacitors[J]. Small, 2018, 14(47): 1-20.
[32]吳煥嶺, 郭慶, 趙杰, 等. 基于細(xì)菌纖維素的高強生物質(zhì)長絲纖維的制備與表征[J]. 絲綢, 2022, 59(10): 34-40.
WU Huanling, GUO Qing, ZHAO Jie, et al. Preparation and characterization of acontinuous biomass filament with high mechanical performance based on bacterial cellulose[J]. Journal of Silk, 2022, 59(10): 34-40.
[33]YANG C, WU Q, XIE W, et al. Copper-coordinated cellulose ion conductors for solid-state batteries[J]. Nature, 2021, 598(7882): 590-596.
[34]DU M, PENG Z, LONG X, et al. Tuning the metal ions of prussian blue analogues in separators to enable high-power lithium metal batteries[J]. Nano Letters, 2022, 22(12): 4861-4869.
[35]MITTAL N, TIEN S, LIZUNDIA E, et al. Hierarchical nanocellulose-based gel polymer electrolytes for stable Na electrode position in sodium ion batteries[J]. Small, 2022, 18(43): 1-12.
[36]CHENG M, QU T, ZI J, et al. A hybrid solid electrolyte for solid-state sodium ion batteries with good cycle performance[J]. Nanotechnology, 2020, 31(42): 1-24.
[37]WANG T, LIU Q, ZHOU J, et al. Natural supra-molecular structure engineering for high-performance potassium batteries separator[J]. Advanced Energy Materials, 2022, 12(44): 1-9.
[38]ZHANG K, PANG Y, CHEN C, et al. Stretchable and conductive cellulose hydrogel electrolytes for flexible and foldable solid-state supercapacitors[J]. Carbohydrate Polymers, 2022, 293: 1-11.
[39]HUANG J, ZHU Y, FENG Y, et al. Research progress on key materials and technologies for secondary batteries[J]. Acta Physico Chimica Sinica, 2022, 38(12): 28-173.
[40]許曉雄, 邱志軍, 官亦標(biāo), 等. 全固態(tài)鋰電池技術(shù)的研究現(xiàn)狀與展望[J]. 儲能科學(xué)與術(shù), 2013, 2(4): 331-341.
XU Xiaoxiong, QIU Zhijun, GUAN Yibiao, et al. All-solid-state lithium-ion batteries: State-of-the-art development and perspective[J]. Energy Storage Science and Technology, 2013, 2(4): 331-341.
[41]WANG T, CHEN H C, YU F, et al. Boosting the cycling stability of transition metal compounds-based supercapacitors[J]. Energy Storage Materials, 2019, 16: 545-573.
[42]劉義波, 李峰, 胡靜. 超級電容器研究進(jìn)展及應(yīng)用分析[J]. 電源技術(shù), 2015, 39(9): 2028-2030.
LIU Yibo, LI Feng, HU Jing. Research progress and application analysis of supercapacitors[J]. Power Supply Technology, 2015, 39(9): 2028-2030.
[43]朱倩瑩, 李瑩蕊, 顧佳俊, 等. 超級電容器生物碳電極的制備及應(yīng)用進(jìn)展[J]. 電源技術(shù), 2020, 44(9): 1395-1398.
ZHU Qianying, LI Yingrui, GU Jiajun, et al. Preparation of supercapacitor biochar electrode and its application[J]. Journal of Power Sources, 2020, 44(9): 1395-1398.
[44]錢宇宸, 楊曉曉, 張晶晶, 等. 超級電容器電極材料的研究進(jìn)展[J]. 東華大學(xué)學(xué)報(自然科學(xué)版), 2022, 48(6): 1-13.
QIAN Yuchen, YANG Xiaoxiao, ZHANG Jingjing, et al. Research progress in electrode materials for supercapacitors[J]. Journal of Donghua University (Natural Science Edition), 2022, 48(6): 1-13.
[45]TAUB A, DE MOOR E, LUO A, et al. Materials for automotive lightweighting[J]. Annual Review of Materials Research, 2019, 49(1): 327-359.
[46]LU Y, LI L, ZHANG Q, et al. Electrolyte and interface engineering for solid-state sodium batteries[J]. Joule, 2018, 2(9): 1747-1770.
[47]XU L, TANG S, CHENG Y, et al. Interfaces in solid-state lithium batteries[J]. Joule, 2018, 2(10): 1991-2015.
[48]QUARTARONE E, MUSTARELLI P. Electrolytes for solid-state lithium rechargeable batteries: Recent advances and perspectives[J]. Chemical Society Reviews, 2011, 40(5): 2525-2540.
[49]YAN M, WANG W P, YIN Y X, et al. Interfacial design for lithium-sulfur batteries: From liquid to solid[J]. Energy Chemistry, 2019, 1(1): 1-32.
[50]WANG S, GAO M, LI Z, et al. Performance evaluation, microbial enzymatic activity and microbial community of a sequencing batch reactor under long-term exposure to cerium dioxide nanoparticles[J]. Bioresour Technol, 2016, 220: 262-270.
[51]YU C, GANAPATHY S, ECK E, et al. Accessing the bottleneck in all-solid state batteries, lithium-ion transport over the solid-electrolyte-electrode interface[J]. Nature Communications, 2017, 8(1): 1-9.
[52]LI C, WANG Z, HE Z, et al. An advance review of solid-state battery: Challenges, progress and prospects[J]. Sustainable Materials and Technologies, 2021, 29: 1-14.
[53]ZHANG T, RAN F. Design strategies of 3D carbon-based electrodes for charge/ion transport in lithium ion battery and sodium ion battery[J]. Advanced Functional Materials, 2021, 31(17): 1-29.
[54]ZHENG F, KOTOBUKI M, SONG S, et al. Review on solid electrolytes for all-solid-state lithium-ion batteries[J]. Journal of Power Sources, 2018, 389: 198-213.
[55]LEWIS J A, CORTES F J Q, BOEBINGER M G, et al. Interphase morphology between a solid-state electrolyte and lithium controls cell failure[J]. ACS Energy Letters, 2019, 4(2): 591-599.
[56]GE X, ZHANG W, SONG F, et al. Single-ion-functionalized nanocellulose membranes enable lean-electrolyte and deeply cycled aqueous zinc-metal batteries[J]. Advanced Functional Materials, 2022, 32(26): 1-11.
[57]ZHU Y, CAO K Y, CHENG W K, et al. A non-Newtonian fluidic cellulose-modified glass microfiber separator for flexible lithium-ion batteries[J]. Ecomat, 2021, 3(4): 1-10.
[58]ZHU G L, ZHAO C Z, PENG H J, et al. A Self-limited free-standing sulfide electrolyte thin film for all-solid-state lithium metal batteries[J]. Advanced Functional Materials, 2021, 31(32): 1-7.
[59]XIE H, YANG C, FU K, et al. Flexible, scalable, and highly conductive garnet-polymer solid electrolyte templated by bacterial cellulose[J]. Advanced Energy Materials, 2018, 8(18): 1-7.
[60]ZHU Y S, XIAO S Y, LI M X, et al. Natural macromolecule based carboxymethyl cellulose as a gel polymer electrolyte with adjustable porosity for lithium ion batteries[J]. Journal of Power Sources, 2015, 288: 368-375.
[61]MA L, CHEN S, WANG D, et al. Super-stretchable zinc-air batteries based on an alkaline-tolerant dual-network hydrogel electrolyte[J]. Advanced Energy Materials, 2019, 9(12): 1-8.
[62]WANG S, ZHANG L, ZENG Q, et al. Cellulose microcrystals with brush-like architectures as flexible all-solid-state polymer electrolyte for lithium-ion battery[J]. ACS Sustainable Chemistry & Engineering, 2020, 8(8): 3200-3207.
[63]彭翔, 黃楷, 孫志杰, 等. 聚合物復(fù)合固體電解質(zhì)材料研究進(jìn)展[J]. 中國材料進(jìn)展, 2020, 39(3): 191-199.
PENG Xiang, HUANG Kai, SUN Zhijie, et al. Progress on the composite solid polymer electrolytes[J]. Advances in Materials in China, 2020, 39(3): 191-199.
[64]LEI Y, DU G, QI Y, et al. Gelation of organic liquid electrolyte to achieve superior sodium-ion full-cells[J]. Journal of Colloid and Interface Science, 2021, 599: 190-197.
[65]LI Y, SUN Z, LIU D, et al. Bacterial cellulose composite solid polymer electrolyte with high tensile strength and lithium dendrite inhibition for long life battery[J]. Energy & Environmental Materials, 2021, 4(3): 434-443.
Abstract: Liquid electrolytes are corrosive, easy to leak, flammable and explosive. Solid electrolytes can act as both electrolyte and separator for energy storage devices, which can effectively solve the bottleneck in the development and application of liquid electrolytes. Compared with other solid electrolyte materials, natural cellulose has the advantages of eco-friendliness, high yield, good stability and excellent mechanical properties, making it a vital biomolecular material. In recent years, the use of cellulose in the solid electrolytes has been extensively researched, and the enhancement mechanism is becoming clear, which has considerable prospects in the application of energy storage.
At present, cellulose-based solid electrolytes have been used in various energy storage devices, and have great potential to improve the devices electrochemical performance. In general, solid electrolytes require high ionic conductivity, stable interface, wide electrochemical window, high mechanical strength, and high environmental stability, so that they are able to assist other components to achieve high performance and cycle stability. As an insulating biomass material, cellulose is a great choice as a raw material for solid electrolytes due to its high stability, good heat resistance, and excellent compatibility. More importantly, cellulose has an easily adjustable pore structure that is beneficial for ion transport and structure design of solid electrolytes.
Cellulose-based solid electrolytes in current research are designed from the two aspects of material and structure. There are usually two strategies to design electrolyte materials, including the modification of cellulose itself and the composite by using other materials. Weakening the celluloses hydrogen bonding network can accelerate the ion transport. In addition, the advantages of cellulose materials can be further exploited by selecting other suitable cellulose as well as other materials corresponding to different requirements. Nevertheless, due to the agglomeration of inactive materials, simple compounding of materials cannot improve the performance of solid electrolytes. Therefore, the structural design has become a more important consideration for the development of cellulose-based solid electrolytes. The structural design of solid electrolytes is related to the morphological structure, aggregated state structure and molecular structure of the cellulose. During the structural design process, the idea of optimizing the internal pore and crystalline structure of cellulose is usually adopted, because it can enhance the electrochemical performance by improving the ion transport pathways owing to the optimized agglomeration and orderliness of the cellulose material in the solid electrolytes.
Changing the stacking of macromolecular chains of cellulose is an important strategy. The higher the proportion of amorphous regions within the cellulose, the more favorable the ion transfer efficiency is. Currently, expanding the spacing between molecular chains to provide fast and efficient ion transport is a hot research topic. Adjusting the aggregated state structure of cellulose, i.e. the proper ratio of crystalline and amorphous regions, is an effective way to improve the electrochemical performance of cellulose-based solid electrolytes while ensuring the mechanical properties of the latter.
The research system on cellulose-based solid electrolytes is maturing now and a large number of valuable results have sprung up. Cellulose as a raw material for solid-state electrolytes has a remarkable effect on the performance enhancement and injects vigor into the booming field of energy storage. However, for cellulose-based solid electrolytes, it is still necessary to solve the problems that the preparation process is not simple enough and that the electrochemical performance and other properties need to be balanced. Unfortunately, many studies on cellulose-based solid electrolytes simply use cellulose as an inactive matrix, while little thought is given to the design of its structure. For this reason, to contribute to the development of solid-state energy storage devices, more in-depth research on the structural design of cellulose for solid electrolytes is necessary in the future.
Key words: natural cellulose; nanocellulose; cellulose structure; material design; structure design; solid electrolyte; energy storage device

