火龍果DREB1D基因的克隆及在轉(zhuǎn)基因擬南芥中的功能分析
張璐芳 侯黔東 蔡曉薇 楊鵾 文曉鵬

摘 ? ?要:【目的】DREB(dehydration responsive element binding)是一類脫水響應(yīng)元件結(jié)合蛋白,在植物響應(yīng)高溫、干旱、高鹽和低溫等多種非生物脅迫過程中發(fā)揮關(guān)鍵作用。以紫紅龍火龍果(Hylocereus monacanthus)為材料,克隆得到DREB轉(zhuǎn)錄因子,并命名為HmDREB1D(HU02G01866.1),探究其生物學(xué)功能。【方法】構(gòu)建HmDREB1D基因植物過表達(dá)載體,通過亞細(xì)胞定位分析HmDREB1D基因在細(xì)胞中的位置。異源轉(zhuǎn)化擬南芥,對(duì)T3代純合系轉(zhuǎn)基因擬南芥(OE3、OE4、OE5)進(jìn)行生物學(xué)功能驗(yàn)證。【結(jié)果】火龍果HmDREB1D基因的開放閱讀框全長723 bp,產(chǎn)生的蛋白定位于細(xì)胞核內(nèi),屬DREB1s亞家族,具有典型的AP2結(jié)構(gòu)域。將HmDREB1D基因轉(zhuǎn)化至擬南芥獲得超表達(dá)轉(zhuǎn)基因株系,與野生型相比,轉(zhuǎn)基因株系表現(xiàn)出較高的抗逆性。在干旱脅迫下,轉(zhuǎn)基因植株T3代純合系種子的萌發(fā)率高于野生型。轉(zhuǎn)基因植株的葉片在逆境脅迫下表現(xiàn)出更低的電導(dǎo)率及更高的保護(hù)性酶活性。實(shí)時(shí)熒光定量PCR分析顯示,RD20、HSP70和COR15A等逆境脅迫響應(yīng)基因在HmDREB1D基因超表達(dá)植株中具有更高的表達(dá)量。【結(jié)論】過表達(dá)HmDREB1D基因通過調(diào)控抗逆相關(guān)基因表達(dá),加速清除植株內(nèi)的活性氧,增強(qiáng)植株的抗逆性。
關(guān)鍵詞:火龍果;HmDREB1D;功能分析;非生物脅迫;亞細(xì)胞定位
中圖分類號(hào):S667.9 文獻(xiàn)標(biāo)志碼:A 文章編號(hào):1009-9980(2023)07-1330-12
Cloning of DREB1D gene from pitaya and its functional analysis in transgenic Arabidopsis thaliana
ZHANG Lufang, HOU Qiandong, CAI Xiaowei, YANG Kun, WEN Xiaopeng*
(College of Life Sciences/Institute of Agro-bioengineering, Guizhou University/Key Laboratory of Plant Resource Conservation and Germplasm Innovation in Mountainous Region (Ministry of Education), Guiyang 550025, Guizhou, China)
Abstract: 【Objective】 DREB (dehydration responsive element binding) proteins are widely present in plants and are primarily involved in the abiotic stress response of plants. The two primary DREB transcription factors are DREB1 and DREB2, with DREB1 being mostly associated to low temperature and drought stress and DREB2 being primarily related to drought, salt, and high temperature stress. Pitaya (Hylocereus monacanthus) belongs to the cactus plants, because of its high nutritional value and strong resistance stress, it is popular with customers in karst regions like Guizhou and Guangxi. DREBs were found responsive to drought stress in pitaya, leaving the underlying mechanism unrevealed. This study intends to clone HmDREB1D (HU02G01866.1) gene and verify its biological function. 【Methods】 The pCambia35s-HmDREB1D-GFP plant overexpression vector was constructed by seamless cloning technology and transformed into Tobacco. The fluorescence signal of HmDREB1D was observed under a laser confocal microscope to determine the subcellular location. The expression vector of pCambia35s-HmDREB1D was constructed. The HmDREB1D gene was transformed into A. thaliana, a total of 6 transgenic A. thaliana plants were obtained, and 3 overexpressed transgenic Arabidopsis (OE3, OE4 and OE5) plants were chosen for further biological verification. After surface sterilization, HmDREB1D transgenic Arabidopsis (OE3, OE4 and OE5) and the wild type Arabidopsis seeds were sown in 250 mmol·L-1 mannitol 1/2 MS medium for drought stress treatment (16 h/8 h day/night cycle, 24 ℃). The germination rate was counted after 7 days. The seedlings grew in 1/2 MS medium for 7 days, and then were transplanted into 1/2 MS medium containing 250 mmol·L-1 mannitol for drought stress treatment (16 h/8 h day/night cycle, 24 ℃). The root length and fresh weight were measured 7 days after the treatment. Transgenic Arabidopsis (OE3, OE4 and OE5) and the wild type Arabidopsis seeds were surface sterilized, sown in 1/2 MS medium and cultured for 7 days, and then transplanted in pots filled with nutrient soil vermiculite (3∶1) and placed in an artificial climate growth chamber for 4 weeks (16 h/8 h day/night light cycle, 24 ℃). Mock drought 20% PEG6000 (3 d), high temperature 42 ℃ (1 d) and low temperature -20 ℃ (1 h) were subsequently applied. Transgenic Arabidopsis and the wild type Arabidopsis leaves were collected before and after stress treatment for determination of physiological and stress gene expression levels. The relative conductivity was measured by Jenco3020. The activities of peroxidase (POD), superoxide dismutase (SOD) and catalase (CAT) were determined by spectrophotometer. The qRT-PCR technique was used to detect the response gene expression level of transgenic Arabidopsis (OE3, OE4 and OE5) and the wild type Arabidopsis under drought (RD20 and RD22), high temperature (HSP70 and HSFA1D) and low temperature (COR47 and COR15A) stress. 【Results】 A 723 bp open reading frame codes the 243 aa HmDREB1D, which is categorized in DREB1s subfamily because of the AP2 domain. The results of GFP fluorescence signal showed that the pCambia35s-HmDREB1D-GFP was transformed into Tobacco and distributed in the nucleus, while the pCambia35s-GFP protein was distributed on the cell surface, indicating that HmDREB1D protein was located in the nucleus. The HmDREB1D gene was transformed into Arabidopsis. The HmDREB1D gene responded greatly to the drought stress with 250 mmol·L-1 mannitol treatments. The results showed that seed germination rate and seedling growth status of transgenic Arabidopsis were better than the wild type Arabidopsis under drought stress. The germination rate of transgenic plant remained above 87.8%, while the germination rate of wild type Arabidopsis was only 37.3%. The results showed that HmDREB1D gene enhanced the tolerance of transgenic Arabidopsis to drought stress by increasing the germination rate of transgenic Arabidopsis under drought stress, indicating that the germination rate was positively correlated with stress resistance. After drought, high temperature and low temperature stress, transgenic Arabidopsis had not only a better phenotype than the wild type Arabidopsis, but also a significantly higher survival rate than the wild type Arabidopsis (p<0.05). The results showed that transgenic Arabidopsis had strong water retention capacity, which improved the survival rate of transgenic Arabidopsis. The relative conductivity of transgenic Arabidopsis was significantly lower than the wild type Arabidopsis (p<0.05). The damage of transgenic Arabidopsis was less than that of the wild type Arabidopsis under drought, high temperature and low temperature stress, indicating that the stress resistance of transgenic Arabidopsis was higher than that of the wild type Arabidopsis. The antioxidant enzyme activity of the HmDREB1D transgenic Arabidopsis was significantly higher than that of the wild type Arabidopsis (p<0.05). The results showed that transgenic Arabidopsis could improve the antioxidant capacity of transgenic Arabidopsis through the activity of antioxidant enzymes, and then reduce the harm caused by drought, high temperature and low temperature stress. According to the results of qRT-PCR, the expression of stress response genes in transgenic Arabidopsis showed upward trend under drought, high temperature and low temperature stress, and was significantly higher than that of the wild type Arabidopsis (p<0.05). The results showed that HmDREB1D gene enhanced the stress ability of transgenic Arabidopsis under drought, high temperature and low temperature stress by inducing the expression of stress-related genes. 【Conclusion】 Aforementioned results suggest that HmDREB1D might be positively involved in the stress response of pitaya.
Key words: Hylocereus monacanthus; HmDREB1D; Functional analysis; Abiotic stress; Subcellular localization
在自然環(huán)境狀態(tài)下,植物遭受著各種非生物脅迫的威脅。非生物脅迫包括干旱、高溫、高鹽和低溫,是影響植物的正常生長和發(fā)育的主要因子[1]。植物通過轉(zhuǎn)錄因子(transcription factor,TF)結(jié)合啟動(dòng)子特異的元件,調(diào)節(jié)功能基因的表達(dá)以響應(yīng)外界信號(hào)[2]。AP2/ERF是一大類植物特異性轉(zhuǎn)錄因子,其特征在于存在穩(wěn)定的AP2結(jié)構(gòu)域。根據(jù)AP2結(jié)構(gòu)域的數(shù)量,AP2/ERF被分為4個(gè)亞家族AP2、DREB、ERF和RVA[3]。其中,DREB(dehydration responsive element-binding protein)是植物特有的一類轉(zhuǎn)錄因子,在逆境應(yīng)答中發(fā)揮重要作用[4]。
近年來,研究人員通過對(duì)光皮樺[5]、玉米[6]和沙冬青AP2/ERF[7]轉(zhuǎn)錄因子家族鑒定與分析發(fā)現(xiàn),DREB類轉(zhuǎn)錄因子參與響應(yīng)干旱、高溫或低溫脅迫的應(yīng)答,這表明DREB可能是植物抗逆相關(guān)的關(guān)鍵調(diào)節(jié)因子。相關(guān)研究發(fā)現(xiàn),DREB蛋白能特異性地識(shí)別共同的核心序列A/GCCGAC的DRE/C重復(fù)(CRT),以調(diào)控下游基因的表達(dá)進(jìn)而增強(qiáng)非生物脅迫耐受性[8]。如苔蘚類BaDBL1通過誘導(dǎo)應(yīng)激響應(yīng)基因的表達(dá),進(jìn)而增強(qiáng)了轉(zhuǎn)基因植物對(duì)干旱和高鹽脅迫的耐受性[9]。胡蘿卜DcDREB1A基因通過提高轉(zhuǎn)基因植物的活性氧清除能力,使得脅迫反應(yīng)基因的表達(dá)上調(diào),正向調(diào)節(jié)了轉(zhuǎn)基因植物的抗旱性[3]。海棠MhDREB2A基因通過調(diào)控脅迫相關(guān)基因的表達(dá),使得轉(zhuǎn)基因植物對(duì)干旱脅迫的耐受性增強(qiáng)[10]。百合LIDREB1G基因過表達(dá)不僅提高了轉(zhuǎn)基因植物的萌發(fā)率和存活率,還促進(jìn)了脅迫相關(guān)基因的表達(dá),使得轉(zhuǎn)基因植物對(duì)干旱、高溫和低溫的耐受性增強(qiáng)[11]。由此推測(cè),不同的植物物種可能是DREB功能存在差異的原因。
火龍果(Hylocereus monacanthus)[12]是貴州、廣西等喀斯特地區(qū)的特色和優(yōu)勢(shì)產(chǎn)業(yè),在農(nóng)業(yè)結(jié)構(gòu)調(diào)整和扶貧開發(fā)等方面發(fā)揮了重要作用。同時(shí),火龍果還具有較高的經(jīng)濟(jì)效益和很強(qiáng)的抗逆性。關(guān)于火龍果抗旱的機(jī)制,前人從功能基因[13]、miRNA[14]和轉(zhuǎn)錄因子[15]等方面開展了研究工作。根據(jù)筆者團(tuán)隊(duì)前期的研究,DREB轉(zhuǎn)錄因子參與了火龍果抗旱響應(yīng)[16]。在此基礎(chǔ)上,筆者在本研究中克隆火龍果DREB1D基因開放閱讀框(open reading frame,ORF)全長序列,通過異源遺傳轉(zhuǎn)化分析其在非生物脅迫應(yīng)答中的表達(dá)特性,并解析其生物學(xué)功能,旨在為深刻認(rèn)識(shí)火龍果抗旱機(jī)制提供信息,也為抗逆遺傳育種提供新資源。
1 材料和方法
1.1 RNA提取及cDNA的合成
以30 d苗齡的紫紅龍組培苗(苗高6~8 cm)為試驗(yàn)材料,利用Plant RNA Kit(Omega,上海)提取試劑盒,參照說明書方法提取肉質(zhì)莖RNA,用1%瓊脂糖凝膠電泳檢測(cè)RNA質(zhì)量,然后用反轉(zhuǎn)錄試劑盒(TaKaRa,日本)合成cDNA第一鏈。
1.2 HmDREB1D克隆和序列分析
在火龍果基因組數(shù)據(jù)庫(http://www.pitayagenomic.com)中查找DREB片段同源序列[17],并命名為HmDREB1D,利用引物設(shè)計(jì)網(wǎng)址(https://crm.vazyme.com/cetool/singlefragment.html)中的方法,設(shè)計(jì)無縫克隆引物(表1)。以cDNA為模板進(jìn)行PCR擴(kuò)增,使用膠回收試劑盒(TaKaRa,日本)對(duì)目的片段回收純化。選用即用型無縫克隆試劑盒(生工,上海)對(duì)將目的片段與pCambia35s-EGFP載體進(jìn)行連接,連接條件為50 ℃、20 min,將重組質(zhì)粒轉(zhuǎn)化于大腸桿菌感受態(tài)細(xì)胞DH5α(全式金,北京),采用PCR技術(shù)鑒定陽性菌落并送上海生物工程有限公司(Sangon)測(cè)序,將構(gòu)建的過表達(dá)載體命名為pCambia35s-HmDREB1D。
對(duì)HmDREB1D蛋白與其他植物中的DREB蛋白序列進(jìn)行多序列比對(duì)和進(jìn)化分析,多序列比對(duì)使用https://espript.ibcp.fr/ESPript/cgi-bin/ESPript.cgi進(jìn)行,利用MEGA11進(jìn)行系統(tǒng)進(jìn)化樹構(gòu)建,設(shè)置Bootstrap為1000次,其他參數(shù)默認(rèn)。
1.3 HmDREB1D基因亞細(xì)胞定位分析
以pCambia35s-EGFP載體為基礎(chǔ),采用無縫克隆技術(shù)克隆HmDREB1D蛋白序列,方法見1.2。設(shè)計(jì)引物去除終止密碼子(表1),構(gòu)建植物過表達(dá)載體pCambia35s-HmDREB1D-GFP。將驗(yàn)證正確的pCambia35s-HmDREB1D-GFP重組質(zhì)粒和pCambia35s-GFP空載質(zhì)粒,采用凍融法分別轉(zhuǎn)入農(nóng)桿菌感受態(tài)GV3101中。參照文獻(xiàn)[18]中的方法,將重懸菌液注射于1月齡的本氏煙草下表皮(背面)。避光培養(yǎng)72 h后,撕取下表皮制片,在激發(fā)波長488 nm和發(fā)射波長510 nm條件下,用激光共聚焦顯微鏡TCS-SP8(Leica 德國)觀察并拍照。
1.4 HmDREB1D基因過表達(dá)擬南芥的產(chǎn)生
采用浸花法[19]浸染野生型擬南芥,收取種子后。在含50 g·L-1潮霉素的1/2 MS培養(yǎng)基中進(jìn)行轉(zhuǎn)基因植株的篩選,利用DNA試劑盒(天根,北京)對(duì)陽性植株進(jìn)行DNA提取,使用特異性引物(表1)對(duì)轉(zhuǎn)基因進(jìn)行PCR驗(yàn)證。
利用Plant RNA Kit提取試劑盒(Omega,上海),提取轉(zhuǎn)基因和野生型擬南芥的RNA,并合成cDNA第一條鏈。使用Primer Premier 5.0軟件設(shè)計(jì)HmDREB1D特異性熒光定量引物(表1),參照Biomarker Fast2×SYBR Green qPCR試劑盒(Thermo Fisher Scientific,北京)進(jìn)行實(shí)時(shí)熒光定量PCR(quantitative real-time PCR,qRT-PCR),表達(dá)水平由梯度熒光定量PCR系統(tǒng)檢測(cè)(Bio-Rad,美國)。設(shè)置3個(gè)生物學(xué)重復(fù)和3次技術(shù)重復(fù),以Actin基因?yàn)閿M南芥內(nèi)參基因(表1)。根據(jù)最終的Ct值,使用2-ΔΔCt的分析方法計(jì)算HmDREB1D基因的表達(dá)水平[20],并挑選高表達(dá)的3個(gè)株系用于后續(xù)功能研究。
1.5 轉(zhuǎn)基因擬南芥非生物脅迫的耐受性評(píng)價(jià)
選用T3代超表達(dá)株系和野生型種子進(jìn)行表面滅菌,對(duì)于萌發(fā)干旱脅迫試驗(yàn),將種子播種于含250 mmol·L-1甘露醇的1/2 MS培養(yǎng)基中,在植物生長室中培養(yǎng),培養(yǎng)條件為16 h(24 ℃),黑暗8 h(20 ℃)。每處理播種30粒,3個(gè)生物學(xué)重復(fù),7 d后統(tǒng)計(jì)萌發(fā)率,萌發(fā)率(%)=種子萌發(fā)數(shù)/供試種子總數(shù)×100。對(duì)于生長特性試驗(yàn),將種子播種于普通1/2 MS培養(yǎng)基中培養(yǎng)7 d,然后轉(zhuǎn)移至含250 mmol·L-1甘露醇的1/2 MS培養(yǎng)基中,每處理20株幼苗,3個(gè)生物學(xué)重復(fù),7 d后測(cè)定其根長度和鮮質(zhì)量。
為了進(jìn)一步分析HmDREB1D轉(zhuǎn)基因植株對(duì)各逆境脅迫的耐受性,選擇5周齡的T3代超表達(dá)和野生型株系,進(jìn)行不同的逆境脅迫處理。對(duì)于干旱脅迫處理,將20% PEG6000溶液從植物根部澆灌3 d,每天50 mL,然后再澆水5 d。對(duì)于高溫脅迫處理,將植物放置42 ℃下處理1 d,正常條件下(24 ℃)恢復(fù)5 d。對(duì)于低溫脅迫處理,植物在-20 ℃下處理1 h,正常條件下(24 ℃)恢復(fù)5 d,統(tǒng)計(jì)存活率。同時(shí),分別在干旱脅迫(3 d)、高溫脅迫(1 d)和低溫脅迫(1 h),采集處理前后植物的葉片用于物理指標(biāo)測(cè)定和應(yīng)激基因表達(dá)分析的模板。
參考電導(dǎo)儀法[21]測(cè)定相對(duì)電導(dǎo)率,采用電導(dǎo)儀Jenco 3020(JENCO,美國)進(jìn)行試驗(yàn)操作,每個(gè)試驗(yàn)3次重復(fù)。計(jì)算公式為:相對(duì)電導(dǎo)率(%)=C1/C2?100(C1:煮沸前的電導(dǎo)率;C2:煮沸后的電導(dǎo)率)。而過氧化物酶(peroxidase,POD)、超氧化物歧化酶(superoxide dismutase,SOD)和過氧化氫酶(catalase,CAT)活性,選擇POD、SOD和CAT檢測(cè)試劑盒(索萊寶,北京),按說明書進(jìn)行測(cè)定。
1.6 應(yīng)激相關(guān)基因表達(dá)分析
選取已經(jīng)被證實(shí)的與應(yīng)激相關(guān)的基因,包括干旱應(yīng)激反應(yīng)基因RD20、RD22[22],熱休克基因HSP70、HSFA1D[23-24]和低溫應(yīng)激基因COR47、COR15A[25],引物序列見表1。收集脅迫處理前后的葉片,提取RNA并合成cDNA,以擬南芥Actin為內(nèi)參基因,采用qRT-PCR技術(shù)檢測(cè)HmDREB1D基因逆境脅迫處理下的表達(dá)水平,檢測(cè)及表達(dá)量分析參照1.4進(jìn)行。
1.7 數(shù)據(jù)統(tǒng)計(jì)學(xué)分析
所有數(shù)據(jù)分析使用Excel 2010軟件統(tǒng)計(jì)并繪制柱形圖,數(shù)據(jù)顯示為3個(gè)獨(dú)立試驗(yàn)的平均值±SE(n=3;生物學(xué)重復(fù))。使用單項(xiàng)方差分析*p<0.05和**p<0.01進(jìn)行統(tǒng)計(jì)分析。
2 結(jié)果與分析
2.1 HmDREB1D基因克隆與序列分析
克隆得到DREB1D基因,并命名為HmDREB1D。通過對(duì)其他植物中的DREB蛋白序列進(jìn)行多序列比對(duì),發(fā)現(xiàn)HmDREB1D基因?qū)儆贏P2超家族,且具有典型的AP2保守結(jié)構(gòu)域(圖1-A)。系統(tǒng)進(jìn)化分析表明,DREB家族轉(zhuǎn)錄因子可分為2個(gè)亞家族(DREB1s和DREB2s)。HmDREB1D蛋白屬于DREB1s亞組,且HmDRE1D蛋白序列與擬南芥AtDRE1D蛋白序列具有高度相似性(圖1-B)。
2.2 HmDREB1D基因過表達(dá)載體構(gòu)建
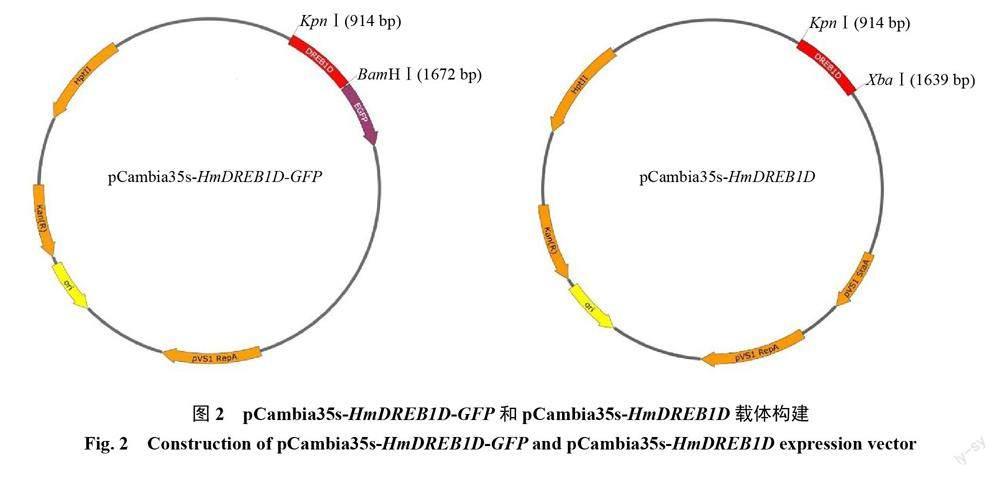
選取測(cè)序正確的菌株進(jìn)行過夜搖菌,提取質(zhì)粒進(jìn)行PCR驗(yàn)證,得到預(yù)期723 bp的基因片段,表明成功構(gòu)建過表達(dá)載體pCambia35s-HmDREB1D-GFP(圖2-A)和pCambia35s-HmDREB1D(圖2-B)。
2.3 HmDREB1D基因亞細(xì)胞定位
為了驗(yàn)證HmDREB1D蛋白在體內(nèi)的亞細(xì)胞位置,將構(gòu)建的pCambia35s-HmDREB1D-GFP載體,通過瞬時(shí)轉(zhuǎn)化注射于煙草葉片中,激光共聚焦顯微鏡跟蹤觀察HmDREB1D蛋白的亞細(xì)胞定位。結(jié)果顯示(圖3),pCambia35s-HmDREB1D-GFP的GFP熒光主要定位于細(xì)胞核,而GFP蛋白均分布于整個(gè)細(xì)胞表面,說明HmDREB1D蛋白定位在細(xì)胞核中,作為轉(zhuǎn)錄因子發(fā)揮作用。
2.4 HmDREB1D擬南芥遺傳轉(zhuǎn)化與生長特性
為了分析HmDREB1D基因的功能,將HmDREB1D基因在擬南芥中過表達(dá),共獲得6株超表達(dá)HmDREB1D轉(zhuǎn)基因擬南芥植物。篩選3株高表達(dá)轉(zhuǎn)基因純合系(OE3、OE4、OE5)(圖4-A)進(jìn)一步分析。
對(duì)T3代超表達(dá)株系幼苗期干旱脅迫耐受性進(jìn)行統(tǒng)計(jì)分析。野生型擬南芥的萌發(fā)率僅為37.3%,而轉(zhuǎn)基因株系的萌發(fā)率保持在87.8%以上(圖4-B~C)。轉(zhuǎn)基因株系的根長度、鮮質(zhì)量均顯著高于野生型(圖4-D~F)。結(jié)果表明,過表達(dá)HmDREB1D基因顯著提高了對(duì)干旱脅迫的耐受性。
2.5 逆境脅迫下超表達(dá)株系的表型分析
選擇5周齡的野生型和轉(zhuǎn)基因擬南芥進(jìn)行干旱和高、低溫脅迫處理(圖5)。結(jié)果表明,在干旱脅迫3 d后,野生型植株發(fā)生明顯皺縮且葉片泛黃,而過表達(dá)HmDREB1D轉(zhuǎn)基因株系葉片沒有發(fā)生明顯的皺縮,且葉片仍是大片綠色;正常澆水5 d后野生型存活率僅為21.8%,而轉(zhuǎn)基因的存活率OE3為44.3%,OE4為82.6%,OE5為64.6%(圖5-A)。在高溫脅迫24 h后,野生型葉片明顯干燥,而大多數(shù)過表達(dá)轉(zhuǎn)基因植株仍然保持新鮮葉片,正常溫度(24 ℃)下恢復(fù)5 d后野生型存活率為20%,而轉(zhuǎn)基因的存活率OE3為43.3%、OE4為82.6%、OE5為61.3%(圖5-B)。在低溫脅迫下1 h后,野生型大部分被凍傷,葉片大多數(shù)變黃或萎蔫,而轉(zhuǎn)基因擬南芥仍保持葉片鮮綠,正常溫度(24 ℃)下恢復(fù)5 d后,野生型存活率為21.7%,而轉(zhuǎn)基因的存活率OE3為52.3%,OE4為84.0%,OE5為66.7%(圖5-C)。過表達(dá)HmDREB1D基因顯著提高了轉(zhuǎn)基因擬南芥在干旱、高溫和低溫脅迫下的存活率。
2.6 逆境下超表達(dá)株系的生理生化反應(yīng)
除了存活率外,還包括生理特征,如相對(duì)電導(dǎo)率和抗氧化活性酶(POD、SOD、CAT)活性的測(cè)定。在正常條件下,野生型與轉(zhuǎn)基因株系之間的生理參數(shù)無顯著差異,而在干旱和高、低溫脅迫條件下,轉(zhuǎn)基因擬南芥中相對(duì)電導(dǎo)率顯著低于野生型(圖6-A),且POD、SOD、CAT的活性顯著高于野生型(圖6-B~D)。這些數(shù)據(jù)表明,過表達(dá)HmDREB1D基因通過提高抗氧化活性,降低細(xì)胞膜損傷程度,進(jìn)而增強(qiáng)了轉(zhuǎn)基因擬南芥抗干旱、高溫和低溫脅迫的能力。
2.7 逆境下超表達(dá)株系應(yīng)激相關(guān)基因的表達(dá)分析
為了了解HmDREB1D基因如何增強(qiáng)對(duì)逆境脅迫響應(yīng)的能力,采用qRT-PCR技術(shù)檢測(cè)轉(zhuǎn)基因株系和野生型中相關(guān)應(yīng)激基因表達(dá)水平(圖7)。結(jié)果顯示,在正常條件下轉(zhuǎn)基因植物中應(yīng)激響應(yīng)基因的表達(dá)水平出現(xiàn)上調(diào),但是在干旱、高溫和低溫脅迫處理下,與野生型相比,轉(zhuǎn)基因植物體內(nèi)干旱(RD20、RD22)、高溫(HSFA1D、HSP70)和低溫(COR47、COR15A)應(yīng)激基因的表達(dá)水平顯著上調(diào)。這表明,HmDREB1D基因通過上調(diào)應(yīng)激相關(guān)基因的表達(dá)來增強(qiáng)轉(zhuǎn)基因植物對(duì)不同非生物脅迫的應(yīng)激能力,從而提高轉(zhuǎn)基因植物抗干旱、高溫和低溫的能力。
3 討 論
本研究從火龍果肉質(zhì)莖中克隆得到HmDREB1D基因,多序列比對(duì)和進(jìn)化分析表明,該基因具有典型的AP2結(jié)構(gòu)域,屬于A1(DREB1)亞組。有研究表明,A1亞組主要參與干旱誘導(dǎo),而不參與低溫誘導(dǎo)[26-27]。但也有研究發(fā)現(xiàn),A1亞組不僅能參與低溫誘導(dǎo),還參與高溫誘導(dǎo)[28-29]。逆境脅迫下,植物的生長狀態(tài)和存活率在一定程度上可以體現(xiàn)出植物對(duì)脅迫環(huán)境的響應(yīng)情況。Ren等[30]研究發(fā)現(xiàn),過表達(dá)AmDREB3轉(zhuǎn)基因植物在干旱、高鹽和高溫脅迫下的生長狀態(tài)和存活率明顯優(yōu)于野生型。本研究中發(fā)現(xiàn),與野生型相比,轉(zhuǎn)基因擬南芥在干旱、高溫和低溫脅迫下表現(xiàn)出更好的生長狀態(tài)和更高的存活率。逆境脅迫下,植物體內(nèi)的相對(duì)電導(dǎo)率與植物抗逆性呈負(fù)相關(guān)[21],本研究中測(cè)得逆境脅迫下轉(zhuǎn)基因擬南芥的相對(duì)電導(dǎo)率顯著低于野生型。正常環(huán)境下,植物體內(nèi)的活性氧清除系統(tǒng)使細(xì)胞內(nèi)活性氧(reactive oxygen species,ROS)保持在較低的水平,以保證植物正常生長[31]。當(dāng)植物受到逆境脅迫時(shí),抗氧化酶活性的增強(qiáng)能有效地幫助植物避免細(xì)胞膜氧化損傷[3],本研究中測(cè)得過表達(dá)HmDREB1D轉(zhuǎn)基因擬南芥在逆境脅迫下體內(nèi)抗氧化酶POD、SOD和CAT的活性均顯著高于野生型。以上研究結(jié)果表明,過表達(dá)HmDREB1D轉(zhuǎn)基因擬南芥通過降低逆境脅迫所產(chǎn)生的傷害,提高了植物在逆境脅迫下的存活率,有效地緩解了逆境脅迫對(duì)膜系統(tǒng)造成的損傷,進(jìn)而提高了植物對(duì)逆境脅迫的抗逆能力。
除了測(cè)定生理指標(biāo),本研究還通過qRT-PCR技術(shù)對(duì)轉(zhuǎn)基因植物進(jìn)行逆境脅迫響應(yīng)基因表達(dá)水平分析,在逆境脅迫下轉(zhuǎn)基因植物與野生型相比表現(xiàn)出更高的表達(dá)水平。相關(guān)研究發(fā)現(xiàn),DREB轉(zhuǎn)錄因子通過與許多逆境脅迫相關(guān)基因的DRE/CRT相互作用,使得相關(guān)基因的表達(dá)上調(diào),從而提高它們?cè)谀婢趁{迫下的表達(dá)能力[32]。苔蘚ScDREB8基因通過上調(diào)干旱、高鹽和熱休克相關(guān)響應(yīng)基因的表達(dá),增強(qiáng)了植物對(duì)逆境脅迫的耐受性[33]。因此,過表達(dá)HmDREB1D基因可能通過提高應(yīng)激響應(yīng)基因的表達(dá)水平,產(chǎn)生各種能抵御植物氧化脅迫的物質(zhì),進(jìn)而增強(qiáng)了植物對(duì)各種逆境脅迫的耐受性。綜上所述,DREB轉(zhuǎn)錄因子對(duì)火龍果逆境脅迫的響應(yīng)具有關(guān)鍵調(diào)控作用,但其分子調(diào)控機(jī)制還需要進(jìn)一步的研究。
4 結(jié) 論
從火龍果中克隆得到的HmDREB1D基因?qū)儆诤硕ㄎ换颉Mㄟ^異源轉(zhuǎn)化擬南芥,驗(yàn)證了HmDREB1D基因激活了一系列逆境響應(yīng)基因的表達(dá),增強(qiáng)了火龍果對(duì)干旱、高溫和低溫脅迫的耐受性。本研究為火龍果抗性研究的分子機(jī)制和新種質(zhì)的創(chuàng)制奠定了基礎(chǔ)。
參考文獻(xiàn) References:
[1] 易家寧,王康才,張琪綺,董雨青,毛曉敏,鄧艷婷. 干旱脅迫對(duì)紫蘇生長及品質(zhì)的影響[J]. 核農(nóng)學(xué)報(bào),2020,34(6):1320-1326.
YI Jianing,WANG Kangcai,ZHANG Qiqi,DONG Yuqing,MAO Xiaomin,DENG Yanting. Effects of drought stress on growth and quality of Perilla frutescens[J]. Journal of Nuclear Agricultural Sciences,2020,34(6):1320-1326.
[2] ZHU J K. Abiotic stress signaling and responses in plants[J]. Cell,2016,167(2):313-324.
[3] LI T,HUANG Y,KHADR A,WANG Y H,XU Z S,XIONG A S. DcDREB1A,a DREB-binding transcription factor from Daucus carota,enhances drought tolerance in transgenic Arabidopsis thaliana and modulates lignin levels by regulating lignin-biosynthesis-related genes[J]. Environmental and Experimental Botany,2020,169:103896.
[4] ZHOU Y X,ZHOU W,LIU H,LIU P,LI Z G. Genome-wide analysis of the soybean DREB gene family:Identification,genomic organization and expression profiles in response to drought stress[J]. Plant Breeding,2020,139(6):1158-1167.
[5] 黃奕孜,錢旺,邱姍,王文新,黃華宏,林二培. 光皮樺AP2/ERF基因家族鑒定與表達(dá)分析[J]. 浙江農(nóng)林大學(xué)學(xué)報(bào),2022,39(6):1183-1193.
HUANG Yizi,QIAN Wang,QIU Shan,WANG Wenxin,HUANG Huahong,LIN Erpei. Identification and expression analysis of AP2/ERF gene family in Betula luminifera[J]. Journal of Zhejiang A & F University,2022,39(6):1183-1193.
[6] 王雷立,董柯清,張嚴(yán)玲,劉青青,王翠玲. 玉米DREB轉(zhuǎn)錄因子家族的全基因組鑒定與分析[J]. 湖南農(nóng)業(yè)大學(xué)學(xué)報(bào)(自然科學(xué)版),2022,48(3):270-281.
WANG Leili,DONG Keqing,ZHANG Yanling,LIU Qingqing,WANG Cuiling. Genome-wide identification and analysis of DREB transcription factor family in maize[J]. Journal of Hunan Agricultural University (Natural Sciences),2022,48(3):270-281.
[7] CAO S L,WANG Y,LI X T,GAO F,F(xiàn)ENG J C,ZHOU Y J. Characterization of the AP2/ERF transcription factor family and expression profiling of DREB subfamily under cold and osmotic stresses in Ammopiptanthus nanus[J]. Plants,2020,9(4):455.
[8] SHARMA V,GOEL P,KUMAR S,SINGH A K. An apple transcription factor,MdDREB76,confers salt and drought tolerance in transgenic tobacco by activating the expression of stress-responsive genes[J]. Plant Cell Reports,2019,38(2):221-241.
[9] LIANG Y Q,LI X S,YANG R R,GAO B,YAO J X,OLIVER M J,ZHANG D Y. BaDBL1,a unique DREB gene from desiccation tolerant moss Bryum argenteum,confers osmotic and salt stress tolerances in transgenic Arabidopsis[J]. Plant Science,2021,313:111047.
[10] 黨明青,王京平,冉昆,劉加芬,李慧峰. 泰山海棠抗旱基因MhDREB2A的克隆與功能鑒定[J]. 沈陽農(nóng)業(yè)大學(xué)學(xué)報(bào),2022,53(4):462-468.
DANG Mingqing,WANG Jingping,RAN Kun,LIU Jiafen,LI Huifeng. Cloning and functional idenfication of drought-resistant related gene in Malus hupehensis[J]. Journal of Shenyang Agricultural University,2022,53(4):462-468.
[11] LIU B J,ZHOU Y,LAN W,ZHOU Q,LI F,CHEN F,BAO M Z,LIU G F. LlDREB1G,a novel DREB subfamily gene from Lilium longiflorum,can enhance transgenic Arabidopsis tolerance to multiple abiotic stresses[J]. Plant Cell,Tissue and Organ Culture (PCTOC),2019,138(3):489-506.
[12] FERNANDES A C F,DE SOUZA A C,RAMOS C L,PEREIRA A A,SCHWAN R F,DIAS D R. Sensorial,antioxidant and antimicrobial evaluation of vinegars from surpluses of Physalis (Physalis pubescens L.) and red pitahaya (Hylocereus monacanthus)[J]. Journal of the Science of Food and Agriculture,2019,99(5):2267-2274.
[13] NIE Q,GAO G L,F(xiàn)AN Q J,QIAO G,WEN X P,LIU T,PENG Z J,CAI Y Q. Isolation and characterization of a catalase gene “HuCAT3” from pitaya (Hylocereus undatus) and its expression under abiotic stress[J]. Gene,2015,563(1):63-71.
[14] LI A L,WEN Z,YANG K,WEN X P. Conserved miR396b-GRF regulation is involved in abiotic stress responses in pitaya (Hylocereus polyrhizus)[J]. International Journal of Molecular Sciences,2019,20(10):2501.
[15] 葛菲,聶瓊,喬光,張婷,吳艷,文曉鵬. 轉(zhuǎn)火龍果過氧化氫酶基因煙草植株的獲得及其抗旱性分析[J]. 西南大學(xué)學(xué)報(bào)(自然科學(xué)版),2016,38(11):57-63.
GE Fei,NIE Qiong,QIAO Guang,ZHANG Ting,WU Yan,WEN Xiaopeng. Gain of the HuCAT genetic tobacco and analysis on drought stress resistance of transgenic tobacco[J]. Journal of Southwest University (Natural Science Edition),2016,38(11):57-63.
[16] 樊慶杰. 火龍果應(yīng)答干旱脅迫的分子基礎(chǔ)[D]. 貴陽:貴州大學(xué),2013.
FAN Qingjie. Molecular basis of Pitaya response to drought stress[D]. Guiyang:Guizhou university,2013.
[17] CHEN J Y,XIE F F,CUI Y Z,CHEN C B,LU W J,HU X D,HUA Q Z,ZHAO J,WU Z J,GAO D,ZHANG Z K,JIANG W K,SUN Q M,HU G B,QIN Y H. A chromosome-scale genome sequence of pitaya (Hylocereus undatus) provides novel insights into the genome evolution and regulation of betalain biosynthesis[J]. Horticulture Research,2021,8(1):164.
[18] ZHENG L P,YAO J N,GAO F L,CHEN L,ZHANG C,LIAN L L,XIE L Y,WU Z J,XIE L H. The subcellular localization and functional analysis of Fibrillarin2,a nucleolar protein in Nicotiana benthamiana[J]. BioMed Research International,2016,2016:2831287
[19] CLOUGH S J,BENT A F. Floral dip:A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana[J]. The Plant Journal,1998,16(6):735-743.
[20] SCHMITTGEN T D,LIVAK K J. Analyzing real-time PCR data by the comparative CT method[J]. Nature Protocols,2008,3(6):1101-1108.
[21] 葛菲. 火龍果CAT基因在煙草中的遺傳轉(zhuǎn)化及功能分析[D]. 貴陽:貴州大學(xué),2016.
GE F. Genetic transformation and functional characterization of pitaya CAT gene in tobacco[D]. Guiyang:Guizhou University,2016.
[22] KANG G J,YAN D,CHEN X L,LI Y,YANG L F,ZENG R Z. Molecular characterization and functional analysis of a novel WRKY transcription factor HbWRKY83 possibly involved in rubber production of Hevea brasiliensis[J]. Plant Physiology and Biochemistry,2020,155:483-493.
[23] NIU X,LUO T L,ZHAO H Y,SU Y L,JI W Q,LI H F. Identification of wheat DREB genes and functional characterization of TaDREB3 in response to abiotic stresses[J]. Gene,2020,740:144514.
[24] LU X,YANG L,YU M Y,LAI J B,WANG C,MCNEIL D,ZHOU M X,YANG C W. A novel Zea mays ssp. mexicana L. MYC-type ICE-like transcription factor gene ZmmICE1,enhances freezing tolerance in transgenic Arabidopsis thaliana[J]. Plant Physiology and Biochemistry,2017,113:78-88.
[25] YANG X Y,WANG R,HU Q L,LI S L,MAO X D,JING H H,ZHAO J T,HU G B,F(xiàn)U J X,LIU C M. DlICE1,a stress-responsive gene from Dimocarpus longan,enhances cold tolerance in transgenic Arabidopsis[J]. Plant Physiology and Biochemistry,2019,142:490-499.
[26] GUTTIKONDA S K,VALLIYODAN B,NEELAKANDAN A K,TRAN L S P,KUMAR R,QUACH T N,VOOTHULURU P,GUTIERREZ-GONZALEZ J J,ALDRICH D L,PALLARDY S G,SHARP R E,HO T H D,NGUYEN H T. Overexpression of AtDREB1D transcription factor improves drought tolerance in soybean[J]. Molecular Biology Reports,2014,41(12):7995-8008.
[27] HAAKE V,COOK D,RIECHMANN J,PINEDA O,THOMASHOW M F,ZHANG J Z. Transcription factor CBF4 is a regulator of drought adaptation in Arabidopsis[J]. Plant Physiology,2002,130(2):639-648.
[28] FENG W Q,LI J,LONG S X,WEI S J. A DREB1 gene from zoysiagrass enhances Arabidopsis tolerance to temperature stresses without growth inhibition[J]. Plant Science,2019,278:20-31.
[29] YANG W,LIU X D,CHI X J,WU C A,LI Y Z,SONG L L,LIU X M,WANG Y F,WANG F W,ZHANG C,LIU Y,ZONG J M,LI H Y. Dwarf apple MbDREB1 enhances plant tolerance to low temperature,drought,and salt stress via both ABA-dependent and ABA-independent pathways[J]. Planta,2011,233(2):219-229.
[30] REN M Y,WANG Z L,XUE M,WANG X F,ZHANG F,ZHANG Y,ZHANG W J,WANG M Y. Correction:Constitutive expression of an A-5 subgroup member in the DREB transcription factor subfamily from Ammopiptanthus mongolicus enhanced abiotic stress tolerance and anthocyanin accumulation in transgenic Arabidopsis[J]. PLoS One,2019,14(12):e0227290.
[31] 徐小艷,姚新轉(zhuǎn),呂立堂,趙德剛. 煙草NtNAC1基因的克隆及其在煙草中的抗旱功能分析[J]. 植物生理學(xué)報(bào),2018,54(6):1085-1094.
XU Xiaoyan,YAO Xinzhuan,L? Litang,ZHAO Degang. Cloning of NtNAC1 gene from Nicotiana tabacum and its analysis of drought-resistant function[J]. Plant Physiology Journal,2018,54(6):1085-1094.
[32] AGARWAL P K,AGARWAL P,REDDY M K,SOPORY S K. Role of DREB transcription factors in abiotic and biotic stress tolerance in plants[J]. Plant Cell Reports,2006,25(12):1263-1274.
[33] LIANG Y Q,LI X S,ZHANG D Y,GAO B,YANG H L,WANG Y C,GUAN K Y,WOOD A J. ScDREB8,a novel A-5 type of DREB gene in the desert moss Syntrichia caninervis,confers salt tolerance to Arabidopsis[J]. Plant Physiology and Biochemistry,2017,120:242-251.

