過渡金屬硼酸鹽K7{(BO3)Zn[B12O18(OH)6]}·H2O的合成與量子化學研究
榮 成蔣 疆 李清祿
(福建農林大學生命科學學院應用化學系,福州 350002)
過渡金屬硼酸鹽K7{(BO3)Zn[B12O18(OH)6]}·H2O的合成與量子化學研究
榮 成*蔣 疆 李清祿
(福建農林大學生命科學學院應用化學系,福州 350002)
通過水熱的方法合成了一例新的過渡金屬硼酸鹽K7{(BO3)Zn[B12O18(OH)6]}·H2O(1),并對其進行了紅外,熱重,能譜以及X-射線單晶衍射和粉末衍射分析。晶體學測試結果表明,化合物1屬于正交晶系,Pmn21空間群,晶胞參數a=1.236 51(4)nm,b=0.909 61(3)nm,c=1.304 05(5)nm,V=1.466 72(9)nm3,Z=2,R1=0.049 7,wR2=0.146 8。對{(BO3)Zn[B12O18(OH)6]}7-陰離子簇的量化分析顯示此簇中的端基BO3基團對前線分子軌道的貢獻最大。除端O原子外,BO3端基的原子電荷較其它B和O原子電荷低。
過渡金屬;硼酸鹽;聚陰離子簇;量子化學
0 Introduction
Borate materials containing transitional metals have attracted much research interest due to their rich structuralchemistry and diverse applications in mineralogy and industry[1-5].From the point of chemistry,boron atoms coordinate with oxygen not only in threefold(triangular,BO3)but also in four-fold coordination(tetrahedral,BO4).These BO3and BO4can further polymerize to all kinds of large oxoborate clusters throughcommoncorners(oxygen atoms).Such polyanion clusters can be considered as the secondary building units(SBUs)to take part in the construction of novel borates.In the past seven decades,borates have formed the following systems:main group and transition metal(TM)borates[6],rare earth borates[7-8],organically[9-11]and TM-complex templated borates[12-14].Wherein,the most borates with framework containing metal were made by temperature solid state method or boric acid flux method.It′s very rare for the metal borates synthesized by the hydrothermal conditions[15].Although people have discovered about 30 borate polyanion clusters,the theoretic research about the electronic structures of the clusters has only attracted relatively little attention[16].In this paper,we report a novel transitional metal borates K7{(BO3)Zn[B12O18(OH)6]}·H2O(1)with framework containing a distorted O2ZnO3square pyramid.Besides,we using density functional theory (DFT)[17]calculated the electronic structure of{(BO3)Zn[B12O18(OH)6]}7-cluster at B3LYP/(6-31G(d)+LanL2DZ)level and natural bond orbital(NBO)populationwasanalyzedundertheGaussian09program.
1 Experimental
1.1 Materials and methods
All chemicals were purchased from commercial sources and used without further purification.The FTIR spectrum (KBr pellet)was recorded on an ABB Bomen MB 102 spectrometer.Thermogravimetric analysis was performed on a Mettler TGA/SDTA 851e analyzer with a heating rate of 10℃·min-1under an air atmosphere.Energy dispersive spectroscopy(EDS)scanning was analyzed by JEOL JSM6700F Field Emission Scanning Electron Microscope(FESEM).PowderX-raydiffraction (PXRD)patternswere collected on a Rigaku DMAX 2500 diffractometer using graphite-monochromatedCu Kα radiation(λ=0.154 18 nm)in the angular range 2θ=5°~65°with a step size of 0.05°.
1.2 Synthesis and characterization
Synthesis of the compound 1 was achieved by a hydrothermal technique in a Teflon-lined stainless steelbomb under synthetic reaction conditions determined empirically.A mixture of Zn(CH3COO)2·2H2O(0.219 g),K2B4O7·4H2O(0.307 g),H2O(1.0 mL)and DMF(5.0 mL)in a molar ratio of about 1∶1∶56∶68 was sealed in a 30 mL stainless reactor with a Teflon liner at 180℃ for 7 d,then cooled to room temperature.Colorless block crystals of 1 were recovered by filtration,washed with distilled water and dried in air,respectively.Yield:40%(based on Zn).
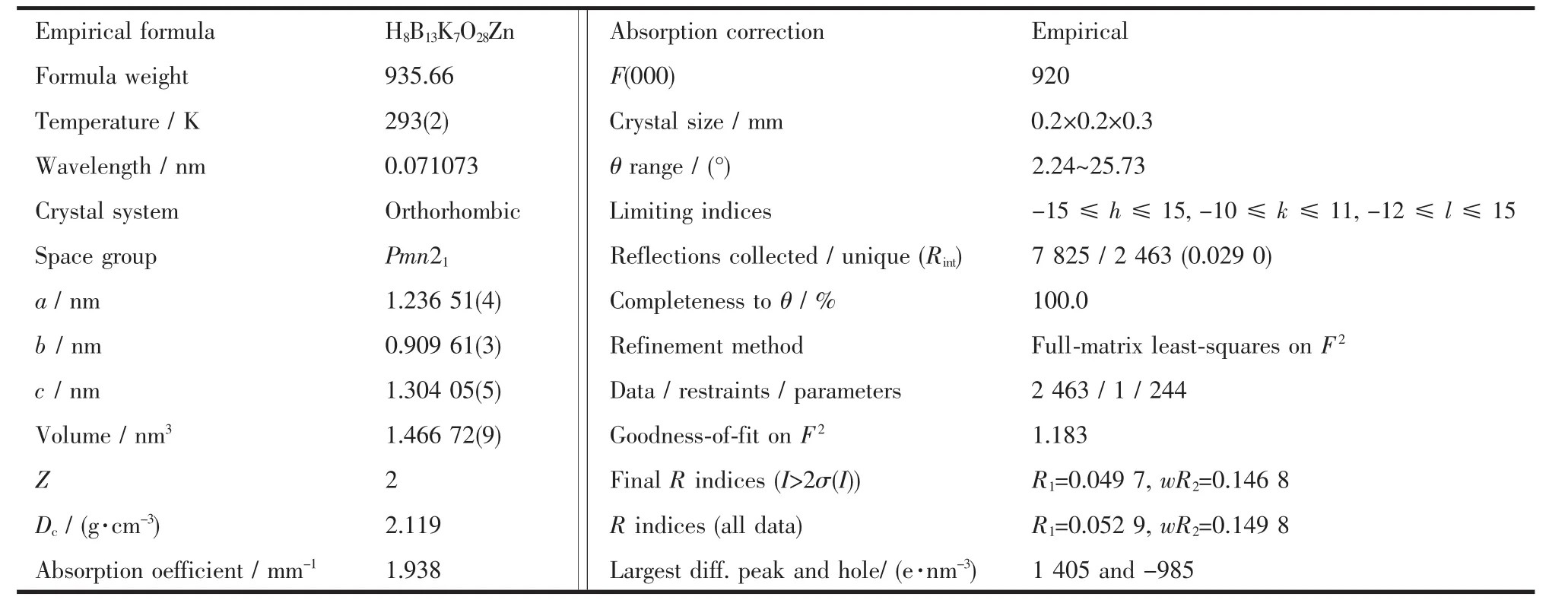
Table 1 Crystal data for compound 1
1.3 Single-crystal structure determination
The intensity data sets were collected on Rigaku Mercury CCD with graphite-monochromated Mo Kα radiation(λ=0.071 073 nm)in the ω scanning mode at room temperature.All absorption corrections were performed using the multiscan program.The structure was solved by direct methods and refined by full-matrix least squares on F2with the SHELX-97 program[18].All nonhydrogen atoms were refined anisotropically.The hydrogen atoms associated with the hydroxyl groups were added in the riding model and refined isotropically.The hydrogen atoms of the water molecule couldn′t be identified because of the disorder of the oxygen atomin the water molecule.Crystal data and structural refinement parameters for 1 are summarized in Table 1 and selected bond lengths and angles are listed in Table 2.
ICSD:424019.
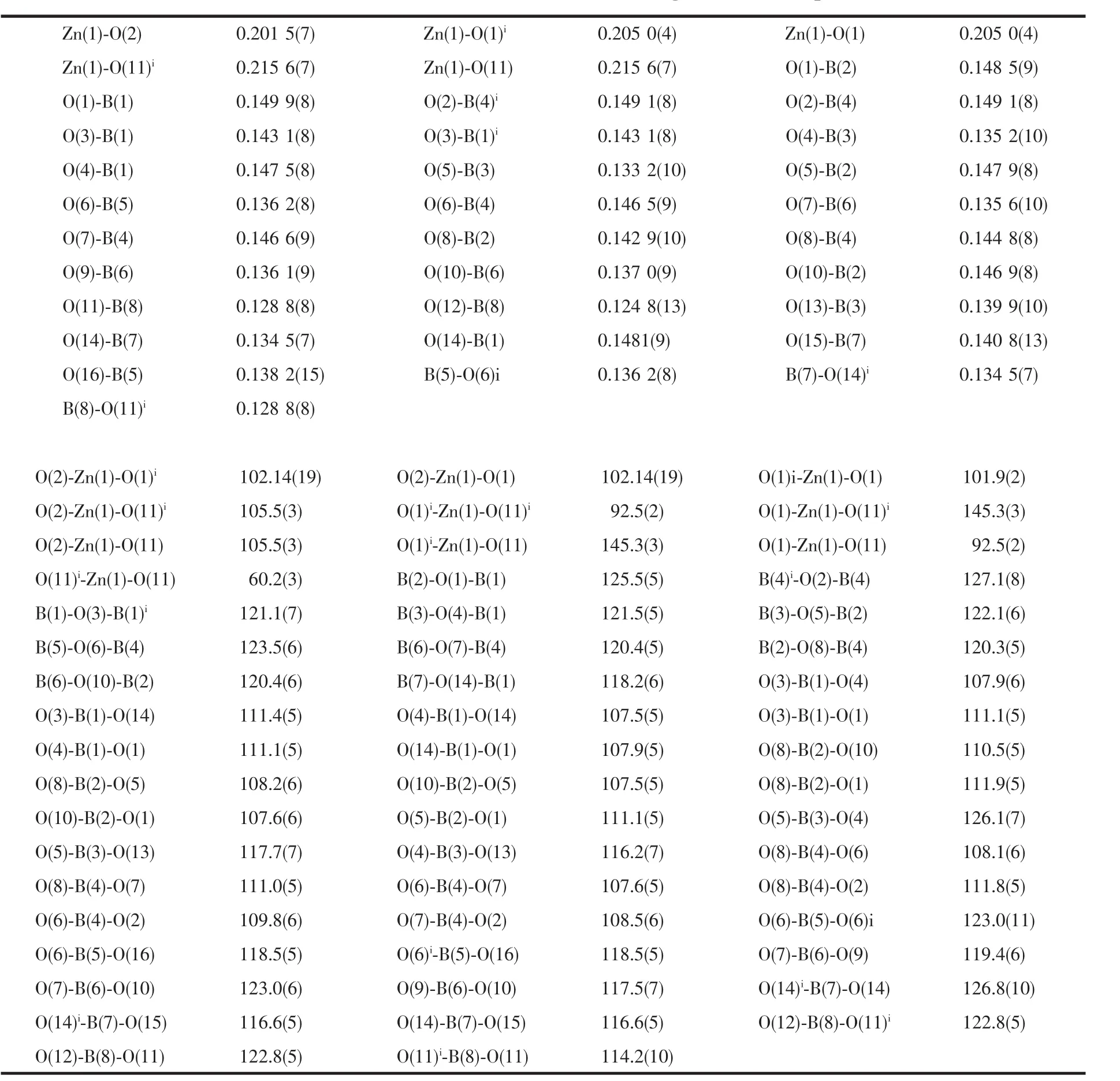
Table 2 Selected bond distances(nm)and bond angles(°)for compound 1
1.4 Computational description
The energy of polyanion{(BO3)Zn[B12O18(OH)6]}7-was calculated at the gradient-correction DFT level using the three-parameter fitofexchange and correlation functionals of Becke (B3LYP)[19],which includes the correlation functional of Lee et al[20].The 6-31G(d)Pople basis set was applied for B,H and O atoms and the LanL2DZ effective core potential basis set was used for Zn atom.Natural bond orbital(NBO)analysis[21-22]was carried out at B3LYP/(6-31G(d)+LanL2DZ)level of theory to reveal the partial charge of atom and donor and acceptor interactions.All computations were done by Gaussian09[23].
2 Results and discussion
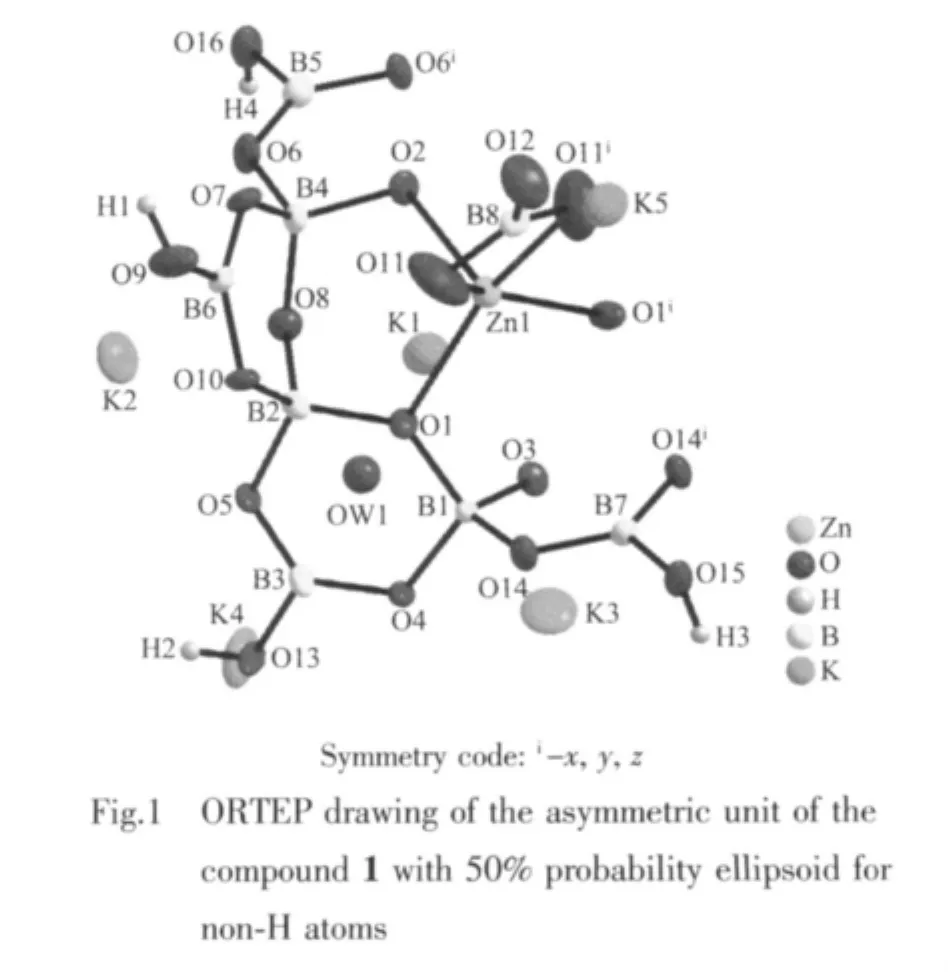
2.1 Description of the structure
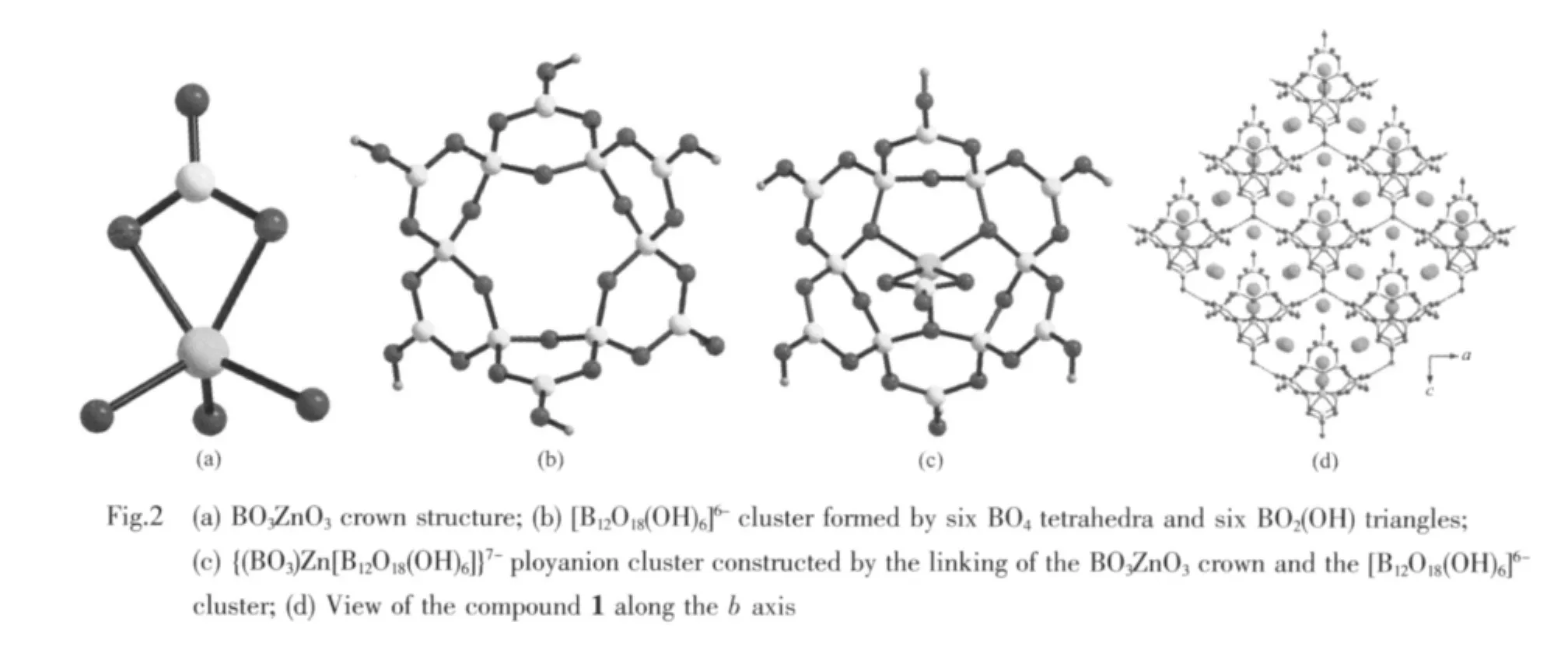
Single-crystal X-ray analysis indicates the asymmetric unit of compound 1 contains 30 independent non-H atoms,including 1 Zn,5 K,7 B and 17 O atoms.As shown in Fig.1,four independent B atoms B3,B5,B6,B7,B8 adopttriangle coordination models.Among them,B3,B5,B6 and B7 are coordinated by two O atoms and one-OH forming BO2(OH)triangles with B-O bond distances vary from 0.133 1 to 0.141 0 nm.Different from the B3,B5,B6 and B7 atoms,the B8 atom is coordinated by two μ2-O atoms and one terminal O atom forming the B(8)O3triangle and the B-O bond lengths varying from 0.124 8 to 0.1286 nm in the B(8)O3triangle,which are significantly shorter than the other B-O bonds in the BO2(OH)triangles.In the asymmetric unit,B1,B2 and B4 atoms are coordinated by four O atoms forming BO4tetrahedra with bond distances varying from 0.143 0 to 0.150 3 nm.The Zn atom is coordinated by three μ3-O atoms with Zn-O bonds changing from 0.2016 to 0.2019 nm and two μ2-O atoms with the same Zn-O bonds 0.2153 nm.The Zn atom and the terminal BO3group are joined together by the sharing-edge linking of two μ2-O atoms forming a crown structure of BO3ZnO3(Fig.2a).Six BO4are linked together by six O vertexes and each adjacent two BO4tetrahedra are bridged by one BO2(OH)triangle to form[B12O18(OH)6]6-polyanion cluster[24](Fig.2b),which is further linked with the BO3ZnO3crown through three μ3-O atoms constructing the{(BO3)Zn[B12O18(OH)6]}7-ployanion cluster(Fig.2c).Then,the{(BO3)Zn[B12O18(OH)6]}7-clusters are further connected by H-bonding interaction into a 2D supermolecular framework.The K+cations reside between adjacent clusters compensating the negative charges of the clusters and hold them together into a 3D structure through bonding with oxygen atoms of the polyanion clusters(Fig.2d).
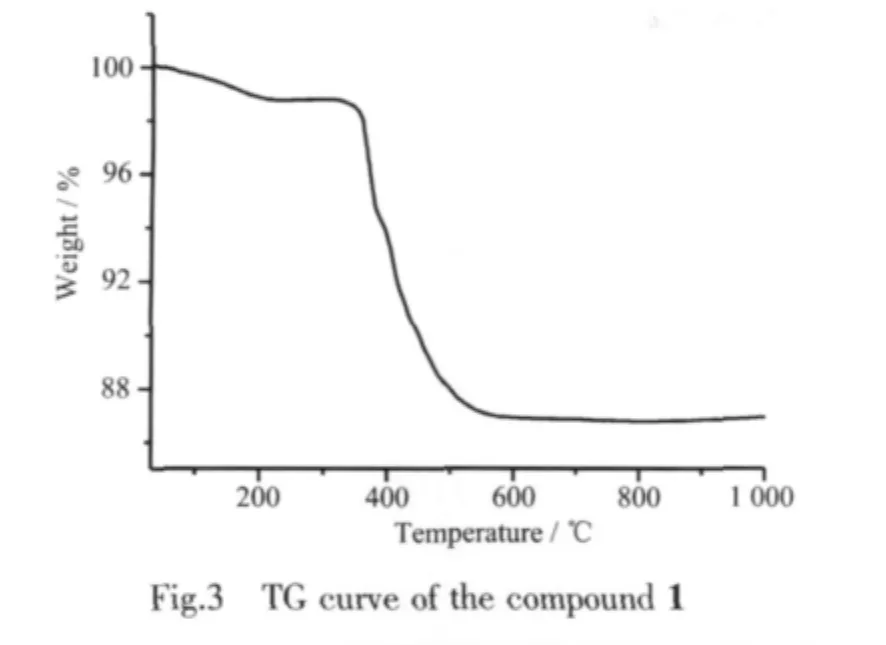
2.2 Thermogravimetric(TG)analysis
TG analysis shows the compound 1 has two step losing water(Fig.3).The loss of 2%weight in the first step from 30 to 360℃corresponds to the release of the free water molecule(Calcd.1.8%).Above 360℃,a graduate weight loss of 11.0%up to 600℃is observed and was assigned to the removal of the hydroxyl groups(Calcd.10.9%).
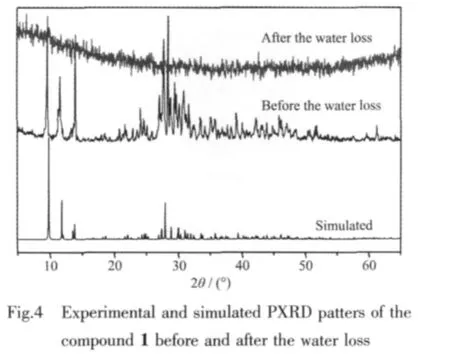
2.3 Powder X-ray diffraction(PXRD)analysis
To investigate the effect of water loss on the structure of the compound 1,the PXRD patterns were recorded before and after water loss of compound 1.As showed in Fig.4,before the water loss,the experimental diffraction peaks of the compound 1 correspond well with those simulated on the basis of single crystal structure,which indicates the structure is stable at room temperature.After heating at 600℃,however,all the diffraction peaks of the remainder disappeared,which confirms the structure of the compound 1 is corrupted after losing water at 600℃.
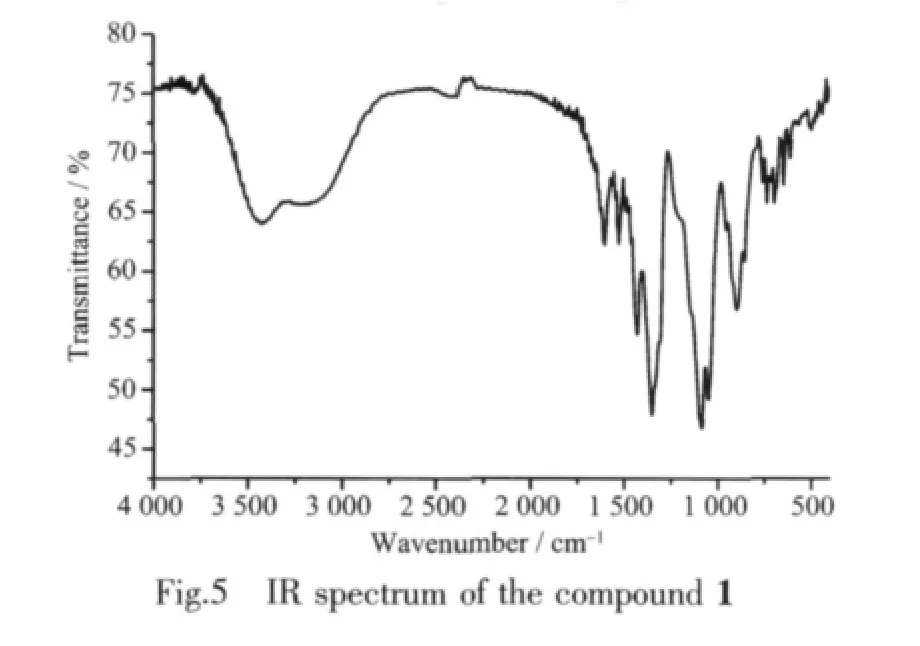
2.3 Infrared(IR)spectrum
The IR spectrum of 1 shows three obviously characteristic peaks at 1 346,1 095 and 890 cm-1corresponding to BO3,BO4and ZnO5groups,respectively(Fig.5).
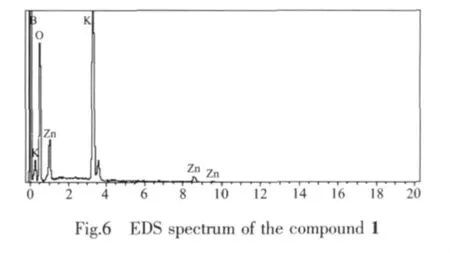
2.4 Energy dispersion spectroscopy(EDS)analysis
The Fig.6 shows the result by EDS analysis.It is further demonstrated that the compound 1 contains B,O,K and Zn atoms.H atom is too light to be detected by EDS.
2.5 Quantum chemistry analysis
The frontier molecular orbitals(FMOs)and nature bond orbitals(NBOs)of the{(BO3)Zn[B12O18(OH)6]}7-ployanion cluster were analyzed through quantum chemistry method.The results show the main orbital components in the highest occupied orbital(HOMO)are concentrated on the terminal B(8)O3group linked with Zn atom.The contribution of the terminal O(12)atom orbital in BO3group to HOMO orbital is 47%.Besides,each O atom of the bridged O(11)and O(11i)provides a 20%contribution to HOMO orbital and the contribution of the B(8)is 11%.Surprising is that the contribution of the Zn to the HOMO is only 3%.Similar with the composition of the HOMO orbital,the main components in the lowest unoccupied orbital(LUMO)come from the three O atoms from the terminal B (8)O3group.The contributions of the terminal O(12)and two bridged O atoms O(11)and O(11i)in the B(8)O3to the LUMO are 28%,34%and 34%,respectively.The contribution of Zn to the LUMO is only 2%.Different from the HOMO,the B(8)atom doesn′t take part in the construction of the LUMO.NBO analysis shows the atomic charges in B(8)O3group has a greatly different from the other atoms in the{(BO3)Zn[B12O18(OH)6]}7-ployanion cluster.The charge of the B(8)in is 0.99,which is remarkable lower than the average charge of 1.28 of the other B atoms.The two bridged O(11)and O(11i)atoms in the B(8)O3have the same charge-0.88 lower the averagecharge-0.99 of the O atoms in[B12O18(OH)6]6-group.The charge of the terminal O(12)in B(8)O3is-0.98 consistent with the average charge.Interestingly,the metal Zn atom has a charge of 1.29,which is identical with the average charge of the B atoms in the[B12O18(OH)6]6-cluster.
3 Conclusions
In summary,a new transitional metal borates K7{(BO3)Zn[B12O18(OH)6]}·H2O constructed with the terminal BO3group,Zn atom,[B12O18(OH)6]6-and counter K cations has been successfully made under hydrothermal conditions.Quantum chemistry calculation for the{(BO3)Zn[B12O18(OH)6]}7-cluster shows the main components of the FMOs come from the contribution of the terminal B(8)O3group.The atom charges except for O(12)in the B(8)O3group are significantly lower than that of the[B12O18(OH)6]6-cluster.
[1]Christ C L,Clark J R.Phys.Chem.Miner.,1977,2:59-87
[2]Burns P C.Can.Mineral.,1995,33:1167-1176
[3]Burns P C,Grice J D,Hawthorne F C.Can.Mineral.,1995,33:1131-1151
[4]Grice J D,Burns P C,Hawthorne F C.Can.Mineral.,1999,37:731-762
[5]Petra B.Adv.Mater.,1998,10:979-992
[6]Touboul M,Penin N,Nowogrocki G.Solid State Sci.,2003,5:1327-1342
[7]Huppertz H,von der Eltz B.J.Am.Chem.Soc.,2002,124:9376-9377
[8]Emme H,Huppertz H.Chem.Eur.J.,2003,9:3623-3633
[9]Wang G M,Sun Y Q,Yang G Y.J.Solid State Chem.,2004,177:4648-4654
[10]Visi M Z,Knobler C B,Owen J J,et al.Cryst.Growth Des.,2006,6:538-545
[11]Wang M S,Guo G C,Chen W T,et al.Angew.Chem.Int.Ed.,2007,46:3909-3911
[12]Wang G M,Sun Y Q,Yang G Y.J.Solid State Chem.,2006,179:1545-1553
[13]Sung H H Y,Wu M,Williams I D.Inorg.Chem.Commun.,2000,3:401-404
[14]Liu Z H,Zhang J J,Zhang W J.Inorg.Chim.Acta,2006,359:519-524
[15]Rong C,Yu Z,Wang Q,et al.Inorg.Chem.,2009,48:3650-3659
[16]ZHA Fu-Biao(查福標),XIE Xian-De(謝先德),LIN Chuan-Yi(林傳易),et al.J.Mineral Petrol(Kuangwu Yanshi),1993,13:1-3
[17]Segall M D,Lindan P J,Probert M J.J.Phys.Condens.Matter.,2002,14:2717-2744
[18]Sheldrick G M.SHELXS-97 and SHELXL97,Program for X-ray Crystal Structure Solution and Refinement,University of G?ttingen,Germany,1997.
[19]Becke A D.J.Chem.Phys.,1993,98:5648-5652
[20]Lee C,Yang W,Parr R G.Phys.Rev.B,1988,37:785-789
[21]Reed A E,Curtiss L A,Weinhold F.Chem.Rev.,1988,88:899-926
[22]Gledening E D,Reed A E,Carpenter J A,et al.NBO,Version 3.1 Ed.
[23]Frisch M J,Trucks G W,Schlegel H B,et al.Gaussian 09,Revision A.02 ed,Gaussian,Inc.,Wallingford CT,2009.
[24]Shakibaie-Moghadam M,Heller G,Timper U.Z.Kristallogr.,1990,190:85-96
Synthesis of Transitional Metal Borate K7{(BO3)Zn[B12O18(OH)6]}·H2O and Quantum Chemistry Study
RONG Cheng*JIANG Jiang LI Qing-Lu
(Department of Applied Chemistry,School of Life Sciences,Fujian Agriculture and Forestry University,Fuzhou 350002,China)
A new transitional metal borate K7{(BO3)Zn[B12O18(OH)6]}·H2O(1)has been synthesized under hydrothermal conditions and characterized by means of IR spectrum,TG,EDS single X-ray and PXRD diffraction.The X-ray crystallography indicates 1 crystallizes in a orthorhombic lattice,Pmn21space group,with a=1.236 51(4)nm,b=0.909 61(3)nm,c=1.304 05(5)nm,V=1.466 72(9)nm3,Z=2,R1=0.049 7,wR2=0.146 8.Quantum chemistry calculation discovers the terminal BO3group has a major contribution to the frontier molecular orbitals(FMOs)of the{(BO3)Zn[B12O18(OH)6]}7-cluster.The atom charges in the terminal BO3group are lower than that of the other B and O atoms,except for the terminal O atom in the BO3group.ICSD:424019.
transitional metal;borate;polyanion cluster;quantum chemistry
O613.8+1;O614.24+1
A
1001-4861(2012)10-2217-06
2012-01-10。收修改稿日期:2012-04-10。
福建教育廳科技基金(No.JA10102)和福建農林大學青年教師基金(No.2010009)資助項目。
*通訊聯系人。E-mail:rongch@fjau.edu.cn;會員登記號:S06N4697M1209。

