基于雙還原體系與膜進樣質譜快速測定15N加富水樣中的方法*
羅 暢 宋國棟 劉素美
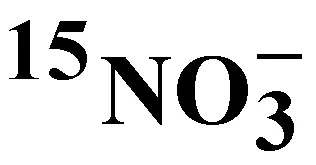
羅 暢1, 2, 3宋國棟1, 2①劉素美1, 2
(1. 中國海洋大學 深海圈層與地球系統前沿科學中心/海洋化學理論與工程技術教育部重點實驗室 山東青島 266100; 2. 青島海洋科學與技術試點國家實驗室 海洋生態與環境科學功能實驗室 山東青島 266237; 3. 中國海洋大學化學化工學院 山東青島 266100)

15N加富樣品; 硝化; 氨基磺酸; 膜進樣質譜
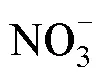
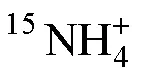
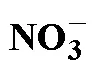
Tab.1 Some main methods, principles, and characteristics for the determination of 15N labeled
1 材料和方法
1.1 測定15N加富樣品中的分析步驟
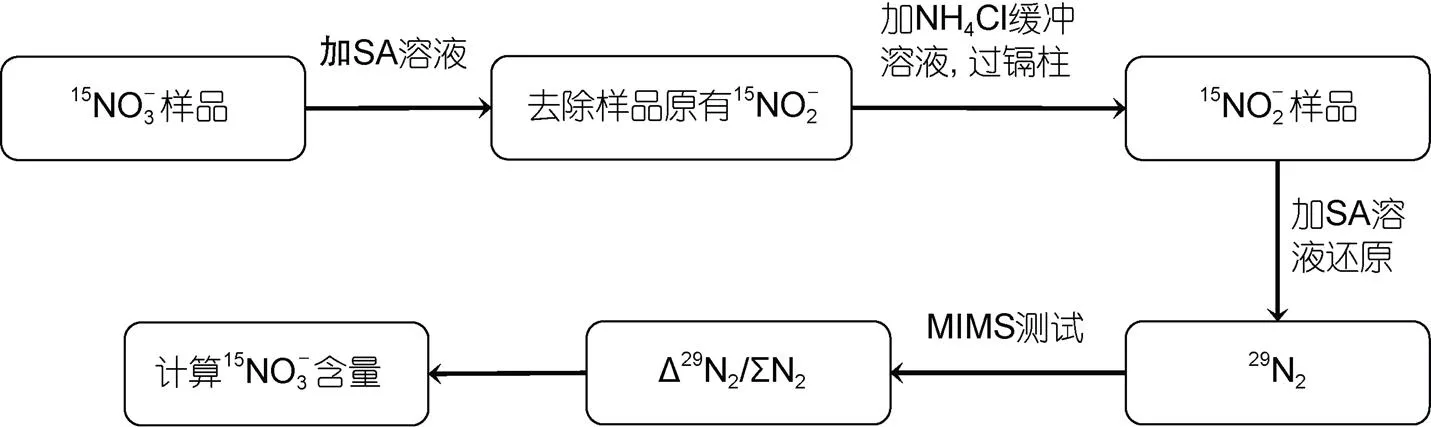
圖1 基于鎘柱與氨基磺酸雙還原體系并結合膜進樣質譜測定15N加富樣品中的簡單流程
整個方法涉及多項因素的條件測試, 影響因素和條件設定如表2, 方法的條件測試具體步驟見1.2~1.5, 方法對樣品的應用測試見1.6。
1.2 鎘柱還原率及還原硝酸鹽濃度范圍測定
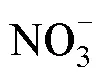
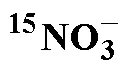
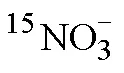
Tab.2 Influencing factors and conditions for determination of by membrane injection mass spectrometry based on cadmium column and sulfamic acid double reduction (SA) system
1.3 氨基磺酸濃度、試劑酸度及反應時間
1.4 線性范圍、精密度及檢測限
檢測限: 以工作曲線線性回歸方程截距標準偏差的3倍除以斜率即檢測限。
1.5 鹽效應
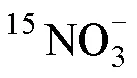
1.6 石老人沙灘沉積物中銨氧化與亞硝酸鹽氧化速率的測定
于2019年5月15日早上08:30 (低潮位時刻)在青島石老人沙灘(36°5′48″N, 120°28′25″E)獲取沉積物柱狀樣, 將表層10 cm沉積物以2 cm的垂向分辨率現場進行分割裝入密封袋中; 獲取沉積物的同時獲取5 L海水, 與分割好的沉積物一同保存在裝有冰盒的保溫箱帶回實驗室進行后續實驗。
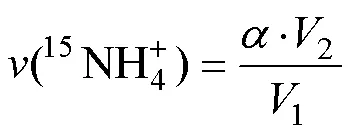
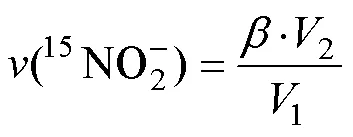
2 結果與討論
2.1 鎘柱還原率
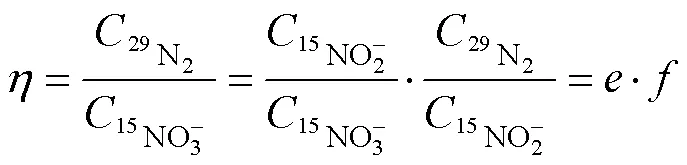
鎘柱還原率≤1, SA還原效率≤1, 為實現轉化率越大, 則需和越接近1。

圖2 過柱(a), 過柱(b), 不過柱(c), 過柱與過柱(d)工作曲線

圖3 不同氨基磺酸(SA)濃度(0.5~20 mmol/L)和酸度(HCl濃度0~1 mol/L)條件下, 轉化為29N2的信號比值Δ29N2/ΣN2變化(a)和轉化率(b)
Fig.3 The signal ratio Δ29N2/ΣN2 variation from to 29N2 (a) and conversion (b) under different sulfamic acid (SA) concentration (0.5~20 mmol/L) and acidity (expressed as HCl concentration 0~1 mol/L)
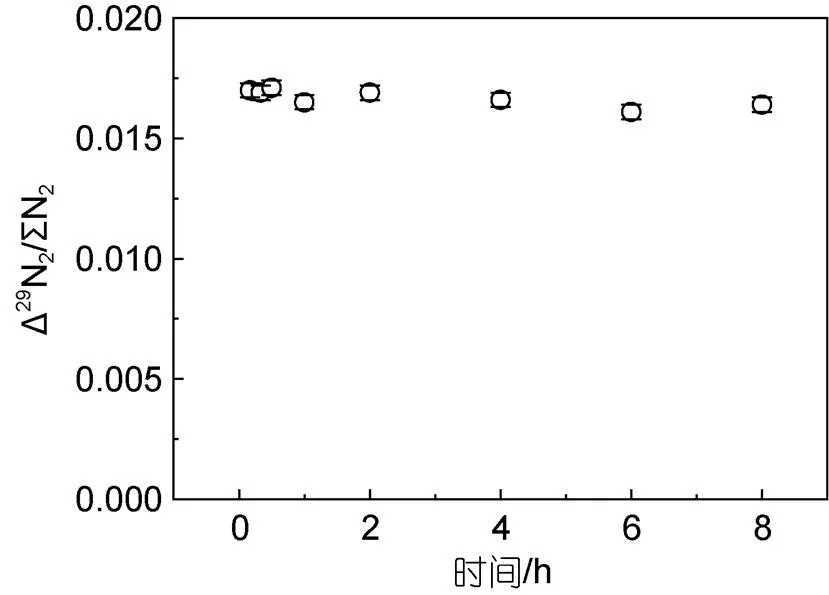
圖4 10 μmol/L經鎘和15 mmol/L SA (1 mol/L HCl)試劑還原后測試信號隨時間的變化
2.3 線性范圍、檢出限和精密度
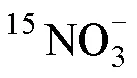
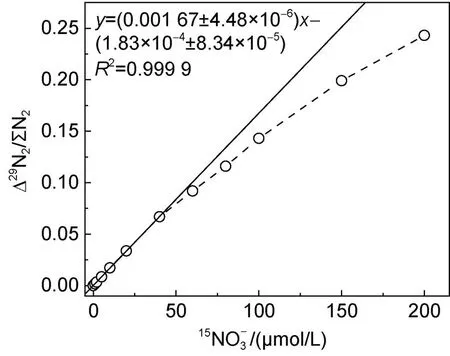
圖5 鎘柱還原-SA反應測定的線性范圍測試
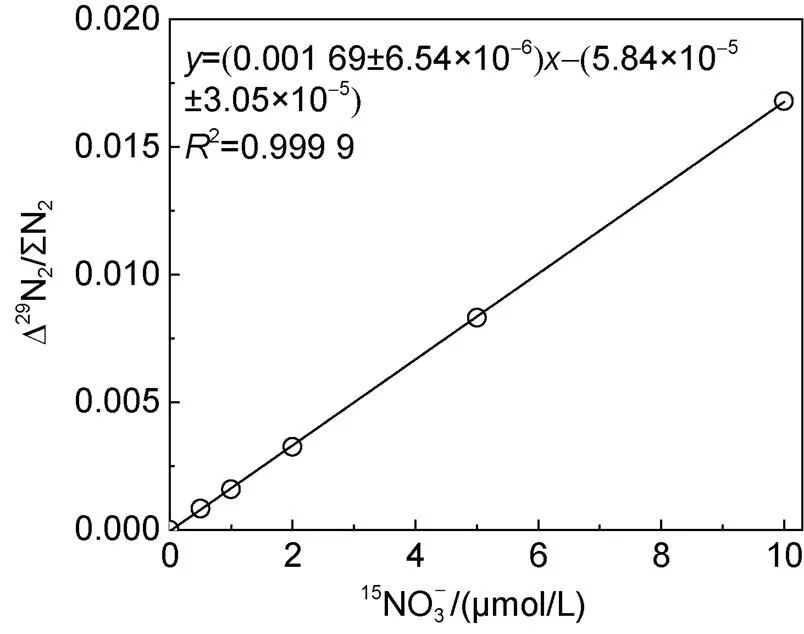
圖6 鎘柱還原-SA反應測定的工作曲線(0~10 μmol/L)
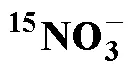
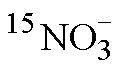
Tab.3 Δ29N2/ΣN2 average measured at 1 and 10 μmol/L of standard and the Δ29N2/ΣN2 standard deviation and Δ29N2/ΣN2 relative standard deviation (n = 4)
2.4 鹽效應影響
通過測定鹽度范圍為0~35的Δ29N2/ΣN2與鹽度為0時Δ29N2/ΣN2信號比值計算相對誤差(圖7), 表明當鹽度為5、30和35, Δ29N2/ΣN2相對誤差明顯低于1%, Δ29N2/ΣN2基本未發生變化; 當鹽度為10~25, Δ29N2/ΣN2相對誤差約為2%, Δ29N2/ΣN2受到的影響也較小。因此, 整個反應過程可視為無顯著的鹽效應。
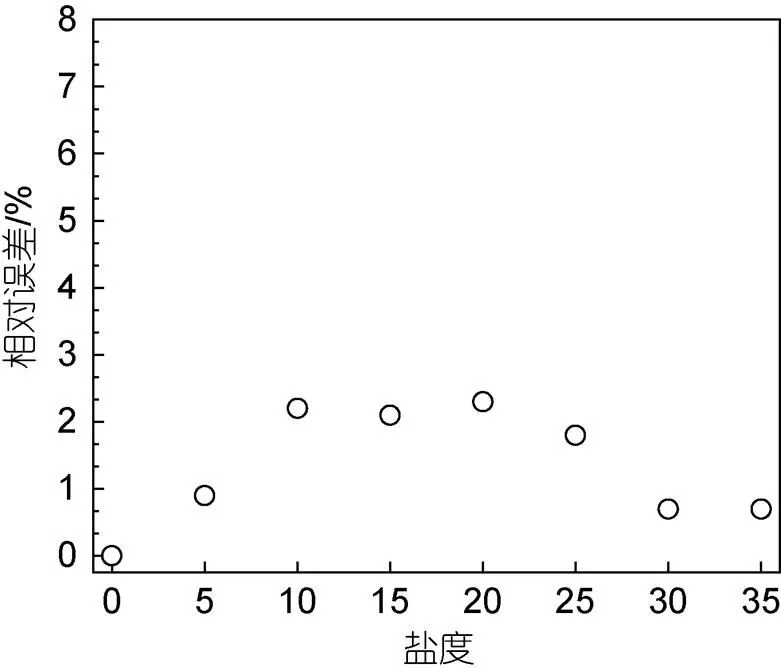
圖7 鹽度為0~35時Δ29N2/ΣN2相對誤差變化
2.5 石老人沙灘沉積物中潛在的銨氧化與亞硝酸鹽氧化速率
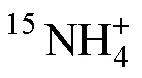
圖8 加富樣品(a)和加富樣品(b)中生成與培養時間的關系
Fig.8 Relationship between production and incubation time in -enriched samples (a) and -enriched samples (b)
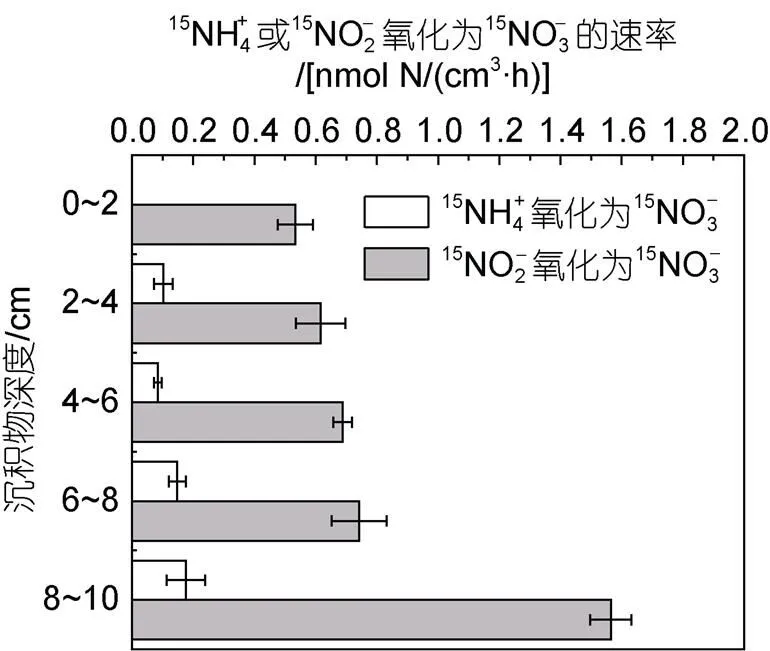
圖9 氧化為及氧化為的速率與沉積物深度的關系
3 結論
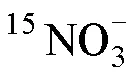
致謝 感謝謝成軍同學對本研究實驗過程與儀器操作所提供的協助; 感謝廣西大學海洋學院寧志銘老師對本文的修改指正。
中華人民共和國國家質量監督檢驗檢疫總局, 中國國家標準化管理委員會, 2008. 海洋監測規范第4部分: 海水分析: GB 17378.4—2007[S]. 北京: 中國標準出版社: 115-117.
杜佳鑫, 杜華超, 王連峰, 2015. 遼東灣河口潮間帶沉積物及附近土壤硝化和反硝化強度研究[J]. 環境保護科學, 41(5): 123-127.
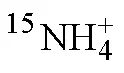
常永凱, 2016. 遼河口沉積物中氨氧化微生物多樣性和硝化作用研究[D]. 大連: 大連海洋大學: 9-12.
謝成軍, 宋國棟, 劉素美, 等, 2020. 自組裝膜進樣質譜系統及其在砂質沉積物異化硝酸鹽還原研究中的應用[J]. 海洋學報, 42(2): 22-29.
BROOKS P D, STARK J M, MCINTEER B B,, 1989. Diffusion method to prepare soil extracts for automated nitrogen-15 analysis [J]. Soil Science Society of America Journal, 53(6): 1707-1711.
CASCIOTTI K L, 2016. Nitrogen and oxygen isotopic studies of the marine nitrogen cycle [J]. Annual Review of Marine Science, 8: 379-407.
CHANG Y K, YIN G Y, HOU L J,, 2021. Nitrogen removal processes coupled with nitrification in coastal sediments off the North East China Sea [J]. Journal of Soils and Sediments, 21(10): 3289-3299.
DAIMS H, LEBEDEVA E V, PJEVAC P,, 2015. Complete nitrification bybacteria [J]. Nature, 528(7583): 504-509.
DEVOL A H, 2015. Denitrification, anammox, and N2production in marine sediments [J]. Annual Review of Marine Science, 7: 403-423.
GARDNER W S, BOOTSMA H A, EVANS C,, 1995. Improved chromatographic analysis of15N:14N ratios in ammonium or nitrate for isotope addition experiments [J]. Marine Chemistry, 48(3/4): 271-282.
GLIBERT P M, MCCARTHY J J, 1984. Uptake and assimilation of ammonium and nitrate by phytoplankton: indices of nutritional status for natural assemblages [J]. Journal of Plankton Research, 6(4): 677-697.
GRANGER J, SIGMAN D M, 2009. Removal of nitrite with sulfamic acid for nitrate N and O isotope analysis with the denitrifier method [J]. Rapid Communications in Mass Spectrometry, 23(23): 3753-3762.
GROFFMAN P M, ALTABET M A, B?HLKE J K,, 2006. Methods for measuring denitrification: diverse approaches to a difficult problem [J]. Ecological Applications, 16(6): 2091-2122.
GRUBER N, GALLOWAY J N, 2008. An Earth-system perspective of the global nitrogen cycle [J]. Nature, 451(7176): 293-296.
HOERING T, 1957. The isotopic composition of the ammonia and the nitrate ion in rain [J]. Geochimica et Cosmochimica Acta, 12(1/2): 97-102.
J?NTTI H, LESKINEN E, STANGE C F,, 2012. Measuring nitrification in sediments-comparison of two techniques and three15NO3?measurement methods [J]. Isotopes in Environmental and Health Studies, 48(2): 313-326.
J?NTTI H, STANGE F, LESKINEN E,, 2011. Seasonal variation in nitrification and nitrate-reduction pathways in coastal sediments in the Gulf of Finland, Baltic Sea [J]. Aquatic Microbial Ecology, 63(2): 171-181.
JENKINS M C, KEMP M W, 1984. The coupling of nitrification and denitrification in two estuarine sediments [J]. Limnology and Oceanography, 29(3): 609-619.
JENSEN K M, JENSEN M H, KRISTENSEN E, 1996. Nitrification and denitrification in Wadden Sea sediments (K?nigshafen, Island of Sylt, Germany) as measured by nitrogen isotope pairing and isotope dilution [J]. Aquatic Microbial Ecology, 11(2): 181-191.
KANA T M, DARKANGELO C, HUNT M D,, 1994. Membrane inlet mass spectrometer for rapid high-precision determination of N2, O2, and Ar in environmental water samples [J]. Analytical Chemistry, 66(23): 4166-4170.
LIN X B, LU K J, HARDISON A K,, 2021. Membrane inlet mass spectrometry method (REOX/MIMS) to measure15N-nitrate in isotope-enrichment experiments [J]. Ecological Indicators, 126(15): 107639.
MCILVIN M R, ALTABET M A, 2005. Chemical conversion of nitrate and nitrite to nitrous oxide for nitrogen and oxygen isotopic analysis in freshwater and seawater [J]. Analytical Chemistry, 77(17): 5589-5595.
MOORE H, 1974. Isotopic measurement of atmospheric nitrogen compounds [J]. Tellus, 26(1/2): 169-174.
MORAES P C, GòMEZ D M A, VINCENZI F,, 2019. Analysis of15N-NO3?via anoxic slurries coupled to MIMS analysis: an application to estimate nitrification by Burrowing Macrofauna [J]. Water, 11(11): 2310.
RUSSOW R, SICH I, STEVENS R J, 1996. Rapid, sensitive and highly selective15N analysis of15N enriched nitrite in water samples and soil extracts by nitric oxide production and CF-QMS measurement [J]. Isotopes in Environmental and Health Studies, 32(4): 323-328.
SIGMAN D M, CASCIOTTI K L, ANDREANI M,, 2001. A bacterial method for the nitrogen isotopic analysis of nitrate in seawater and freshwater [J]. Analytical Chemistry, 73(17): 4145-4153.
SILVA S R, KENDALL C, WILKISON D H,, 2000. A new method for collection of nitrate from fresh water and the analysis of nitrogen and oxygen isotope ratios [J]. Journal of Hydrology, 228(1/2): 22-36.
SONG G D, LIU S M, MARCHANT H,, 2013. Anammox, denitrification and dissimilatory nitrate reduction to ammonium in the East China Sea sediment [J]. Biogeosciences, 10(11): 6851-6864.
STANGE C F, SPOTT O, APELT B,, 2007. Automated and rapid online determination of15N abundance and concentration of ammonium, nitrite, or nitrate in aqueous samples by the SPINMAS technique [J]. Isotopes in Environmental and Health Studies, 43(3): 227-236.
TU Y, FANG Y T, LIU D W,, 2016. Modifications to the azide method for nitrate isotope analysis [J]. Rapid Communications in Mass Spectrometry, 30(10): 1213-1222.
VAN KESSEL M A H J, SPETH D R, ALBERTSEN M,, 2015. Complete nitrification by a single microorganism [J]. Nature, 528(7583): 555-559.
XING M, LIU W G, 2011. An improved method of ion exchange for nitrogen isotope analysis of water nitrate [J]. Analytica Chimica Acta, 686(1/2): 107-114.
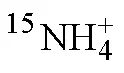
ZHANG X N, WARD B B, SIGMAN D M, 2020. Global nitrogen cycle: critical enzymes, organisms, and processes for nitrogen budgets and dynamics [J]. Chemical Reviews, 120(12): 5308-5351.
LUO Chang1,2,3, SONG Guo-Dong1, 2, LIU Su-Mei1, 2
(1. Frontiers Science Center of Deep Ocean Multispheres and Earth System/Key Laboratory of Marine Chemistry Theory and Technology, Ministry of Education, Ocean University of China, Qingdao 266100, China; 2.Laboratory for Marine Ecology and Environmental Science, Pilot National Laboratory for Marine Science and Technology (Qingdao), Qingdao 266237, China; 3. College of Chemistry and Chemical Engineering, Ocean University of China, Qingdao 266100, China)
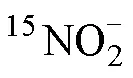
15N enriched sample; nitrification; sulfamic acid; membrane injection mass spectrometry
*國家自然科學基金, U1806211號, 42076035號, 41606093號; 中國海洋大學中央高校基本科研業務, 202072001號。羅暢, 碩士研究生, E-mail: 1051392235@qq.com
宋國棟, 副教授, E-mail: gsong@ouc.edu.cn
2021-10-09,
2021-12-02
P734
10.11693/hyhz20211000237
——MTA教育中心

