HBV相關腎小球腎炎的研究進展
李朝霞 李楠 辛桂杰
摘要:慢性HBV感染人群數量龐大,HBV不僅損害肝臟,腎臟也是主要受累器官之一,HBV相關腎小球腎炎(HBV-GN)是由HBV感染引起的繼發性腎小球腎炎,是HBV感染最常見的肝外并發癥之一。HBV-GN多見于兒童及中青年人,男性多見,臨床上以不同程度蛋白尿為主要表現,可伴有血尿和高血壓,最常見病理類型為膜性腎病,其次為系膜增生性腎小球腎炎和IgA腎病。HBV-GN起病隱匿,缺乏特征性的癥狀及病理學表現,易與各種腎小球腎炎混淆,導致漏診誤診。HBV-GN發病機制復雜,涉及免疫紊亂、病毒直接損傷、遺傳等諸多環節,其中免疫復合物沉積學說已得到廣泛認可。近年來關于HBV-GN發病機制、診斷及治療方面的研究取得了一些重要進展。本文將對此進行綜述,希望為臨床診治提供參考。
關鍵詞:腎小球腎炎; 肝炎病毒,?? 乙型; 診斷; 治療學
基金項目:吉林省衛生廳重點實驗室項目(2018J043)
Research advances in hepatitis B virus-associated glomerulonephritis
LI Zhaoxia, LI Nan, XIN Guijie. (Department of Hepatology, The First Hospital of Jilin University, Changchun 130021, China)
Corresponding author:
XIN Guijie, xingj@jlu.edu.cn (ORCID:0000-0002-0868-0317)
Abstract:
There is are large number of patients with chronic hepatitis B virus (HBV) infection. HBV not only damages the liver, but also involves the kidney. Hepatitis B virus-associated glomerulonephritis (HBV-GN) is secondary glomerulonephritis caused by HBV infection, and it is one of the most common extrahepatic complications of HBV infection. HBV-GN is mainly observed in children and young and middle-aged adults, with varying degrees of proteinuria as the main clinical manifestation, and it may be accompanied by hematuria and hypertension. Membranous nephropathy is the most common pathological type, followed by membrano-proliferative glomerulonephritis and IgA nephropathy. HBV-GN has an insidious onset and lacks characteristic symptoms and pathological manifestations, and thus it may be easily confused with various types of glomerulonephritis, which may lead to missed diagnosis and misdiagnosis. HBV-GN has a complex pathogenesis involving various links such as immune disorders, direct viral damage, and genetics, among which the theory of immune complex deposition has been widely recognized. In recent years, some important advances have been made in the research on the pathogenesis, diagnosis, and treatment of HBV-GN. This article summarizes the above issues, so as to provide a reference for clinical diagnosis and treatment.
Key words:
Glomerulonephritis; Hepatitis B Virus; Diagnosis; Therapeutics
Research funding:
Jilin Province Health Department Key Laboratory Program (2018J043)
據統計,全球慢性HBV感染者數量約為2.4億,其中8%~20%伴有腎功能受損[1-2],主要包括HBV相關腎小球腎炎(HBV-associated glomendonephrits,HBV-GN)。黃瑤等[3]的研究回顧分析了9310例腎臟病理資料,包括繼發性腎小球腎炎(secondary glomerulonephritis,SGN)2523例,其中HBV-GN占10.62%,是較為常見的SGN類型之一。HBV-GN由Combes[4]于1971年首次報道,于1989年正式命名,兒童發病多見,成人發病率男性高于女性,臨床上主要表現為不同程度蛋白尿,可伴有血尿和高血壓,常見病理類型有:膜性腎病(membranous nephropathy,MN)、系膜增生性腎小球腎炎(membrano-proliferative glomerulonephritis,MPGN)和IgA腎病,MN最為常見。
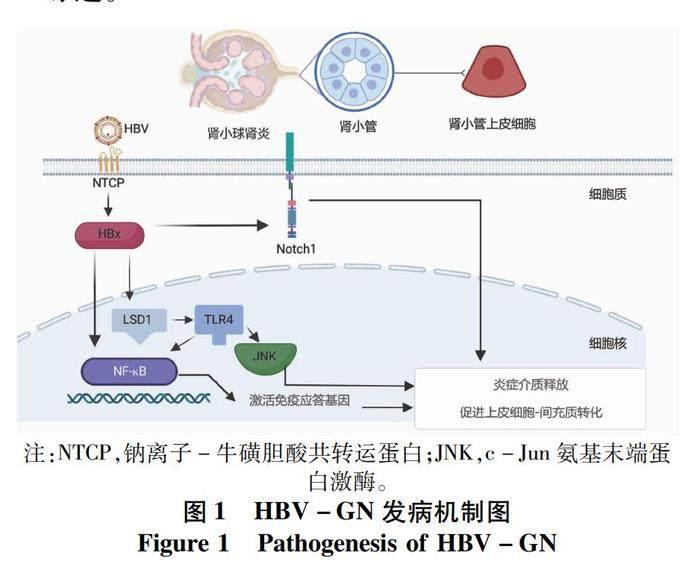
HBV-GN的發病機制尚不清晰,目前普遍認為免疫復合物沉積是其主要發病機制,近年來研究提示病毒直接損傷、免疫損傷及遺傳因素也起到一定作用(圖1)。直至目前,HBV-GN尚無可行性較高的診斷及療效評估指標,亦缺乏確切有效的治療方案。本文就HBV-GN的發病機制、診斷標準及治療進展作一綜述。
1 HBV-GN的發病機制
1.1 病毒抗原與宿主抗體免疫復合物沉積 HBV抗原能夠與自身抗體結合形成免疫復合物沉積于腎小球,因HBV抗原的分子量及電荷屬性不同,沉積部位也不同,具有多種病理學表現。HBeAg大小為17 kD,是三種抗原中最小的一種,可穿透基底膜而沉積于腎小球上皮細胞下,病理可表現為MN。HBsAg和HBcAg大小分別為35~50 kD和22 kD,同屬陰離子,無法穿透腎小球基底膜而沉積于腎小球內皮細胞下及系膜區,病理多表現為MPGN。
1.2 HBV直接損傷腎小管細胞 鈉離子-牛磺膽酸共轉運蛋白(sodium-taurocholate cotransporting polypeptide,NTCP)是一種可在腎臟中表達的糖蛋白[5],最早被認為是膽汁酸運輸的受體并在膽汁酸腸肝循環中起到重要作用[6],后被發現可作為受體與HBV前S1蛋白(HBV preS1)結合介導HBV進入肝細胞[7],初步證實HBV具有感染腎臟的基本條件,但關鍵轉錄因子的表達尚未被證實。多項研究[8-9]觀察到了血清學HBV標志物陰性的HBV-GN患者,推測HBV或可直接損傷腎臟。HBx是一種多功能調節蛋白,具有調節細胞周期及激活信號轉導通路的作用[10-11],與HBV在細胞中的復制密切相關。HBx在腎小管細胞中能夠調節細胞周期蛋白表達從而影響腎小管上皮細胞凋亡[12]。Li等[13]將HBx質粒轉染到人近端腎小管上皮細胞(HK-2),發現HBx質粒轉染增加了NF-κB磷酸化,猜想HBx可能通過激活NF-κB信號通路促進腎小管上皮細胞-間充質細胞轉分化,最終導致腎臟間質纖維化。HBx還可上調組蛋白賴氨酸特異性脫甲基酶(lysine-specific demethylase 1,LSD1),LSD1處于NF-κB上游,通過LSD1-Toll樣受體(TLR)4-NF-κB/JNK信號通路激發炎癥反應,促進炎性介質釋放并誘導細胞凋亡[14-15]。
1.3 病毒介導的特異性免疫效應機制 HBV介導的免疫效應在HBV-GN的發病過程中同樣具有重要作用。Zhou等[16]在HBV-GN的腎組織中檢測到了CD4+、CD8+T淋巴細胞和巨噬細胞,并觀察到了腎小管間質纖維化,提示免疫細胞激活引起的自身細胞損傷在發病過程中具有一定作用。HK-2是非專業的抗原呈遞細胞,正常情況僅表達少量的主要組織相容性復合體Ⅱ(MHC-Ⅱ)類抗原及部分T淋巴細胞、巨噬細胞的共刺激分子,在HBV感染時可轉化為專業的抗原呈遞細胞[17],分泌多種細胞因子并促進免疫炎癥反應[18-19]。HK-2細胞在HBx轉染后可促進CD4+T淋巴細胞的增殖及趨化因子單核細胞趨化蛋白-1的產生,還可使巨噬細胞黏附指數增加,大量激活巨噬細胞并促進炎癥損傷[20]。
Notch1作為Notch信號通路主要受體之一可在HBV-GN患者的腎小管上皮細胞及間質區域表達,隨著腎小管炎癥及間質纖維化加重,受體數量也相應增加。Wang等[21]應用短發夾RNA敲除Notch1可抑制HBx轉染后的腎小管上皮細胞-間充質細胞轉分化,這表明HBV感染通過某種機制上調了Notch1的表達并促進了腎間質纖維化。Notch1還可促進IL-4分泌并減少IFNγ分泌,使輔助性T淋巴細胞1/2(Th1/Th2)細胞增殖失衡,進一步誘發免疫紊亂。
黑色素瘤缺乏因子(absent in melanoma,AIM)2屬HIN-200(hematopoietic IFN-inducible nuclear protein containing a 200-amino-acid repeat)蛋白家族,與細胞質雙鏈DNA結合后能夠促進含半胱氨酸的天冬氨酸蛋白水解酶-1(caspase-1)、IL-1β和IL-18的釋放。Zhen等[22]的研究發現,AIM2在HBV-GN患者腎組織中的表達(81.4%)顯著高于慢性腎小球腎炎患者(4.0%),并與IL-1β、IL-18及caspase-1的
表達密切相關。推測AIM2能夠與HBV DNA結合并激活上述炎癥信號通路,通過免疫介質的釋放增加引起腎損傷。
TLR4是參與機體免疫應答的重要蛋白分子,可調控炎癥細胞及T淋巴細胞活化,還可促進細胞因子釋放并介導免疫炎癥反應。Zhou等[16]研究結果顯示,在HBV-GN患者中,TLR4主要在腎小管和間質間隙中表達并與腎臟病變嚴重程度呈正相關,進一步行體外研究發現TLR4上調可抑制HBV DNA復制,但過強的免疫應答可引起腎臟免疫損傷并促進HBV-GN進展。
1.4 遺傳因素 HLA基因復合體是調節免疫應答的重要基因群,在抗原識別、提呈等方面發揮重要作用,包括Ⅰ、Ⅱ、Ⅲ 3類。Bhimma等[23]在30例HBV-GN黑人兒童腎組織中進行了HLA檢測,發現DQB1*0603的表達頻率顯著高于健康對照組。Park等[24]的研究發現DRB1*1501與DRB1*1502在部分HBV-GN患者中表達頻率明顯增加,其中DRB1*1502 與 HBV-MPGN 呈強相關性,DRB1*1501 與 HBV-MN 呈弱相關性。此外,在部分HBV-GN患者中發現了單點HBx基因突變,提示HBx蛋白或可介導調控區氨基酸的替換,進而影響HBV復制[25]。HBV-GN的發生需要病毒及宿主的遺傳因素共同作用,但相關研究較少,具體機制仍需進一步探索。
2 HBV-GN的診斷
HBV-GN診斷的金標準為腎病理學檢查,以腎組織中檢測到HBV-Ag陽性為基本條件,血清HBV相關指標陽性及除外其他SGN可進一步支持HBV-GN的診斷。腎穿刺活檢屬于有創性檢查,部分患者因禁忌證無法進行,某些地區或基層醫院亦尚未開展該項檢查,臨床上需要可行性較高的無創輔助診斷指標。
M型磷脂酶A2受體(phospholipase A2 receptor,PLA2R)是特發性MN血清學診斷的金標準[26],正常情況下可少量存在于足細胞表面,能夠與血液循環中的抗PLA2R抗體結合形成原位免疫復合物并沉積于腎小球基底膜,通過激活補體途徑損傷腎小球濾過屏障[27]。Xie等[28]應用免疫熒光技術測定了39例HBV-GN患者腎組織中的PLA2R,結果顯示在25例(64%)HBV-GN患者中檢測到了PLA2R,提示PLA2R抗體在HBV-GN中同樣具有診斷價值。同樣地,Dong等[29]測定了103例HBV-GN患者腎組織中的PLA2R,其中有66例(64%)檢測到了PLA2R,并且這部分患者具有更高的尿蛋白水平及更為嚴重的腎臟病理損傷。涂天琪等[30]應用ELISA法測定了229例HBV-GN患者血清抗PLA2R滴度,結果表明抗PLA2R滴度與24 h尿蛋白定量呈正相關,與血白蛋白呈負相關,抗體滴度下降早于24 h尿蛋白下降。血清PLA2R測定對HBV-GN的診斷價值仍需大規模研究進一步驗證。
3 HBV-GN的治療
目前HBV-GN的治療主要為抗病毒及免疫抑制治療。現有研究表明,隨著HBV復制減弱及HBV抗原清除,HBV-GN患者蛋白尿水平及腎功能均可得到顯著改善。2012年KDIGO指南[31]推薦將抗病毒治療作為主要治療手段,包括干擾素(IFN)和核苷酸類似物(NUC)。
3.1 IFN IFN具有抗病毒、抗增殖及免疫調節作用,其HBeAg及HBsAg陰轉率較NUC更高,治療周期更短,適用于兒童及年輕患者。普通IFNα的推薦療程為24周,PEG-IFN的推薦療程為48周,血清轉換率可達15%~46%[32-33]。臨床上應遵循個體化原則評估最佳治療方案。一項納入182例HBV-GN患者的Meta分析[34]結果顯示,IFN在兒童患者中治療效果更確切,與NUC相比,能夠更大程度上緩解蛋白尿水平并提高HBeAg清除率。IFN可擴大HBV感染后免疫機制介導的肝損傷作用,還可引起流感樣綜合征、胃腸道反應、骨髓抑制等不良反應,因此應用受限。
3.2 NUC NUC具有給藥方便、耐受性好等優勢,現已廣泛應用于HBV感染的治療,但部分NUC本身即具有腎毒性,主要包括阿德福韋酯(ADV)及富馬酸替諾福韋酯(TDF)。Udompap等[35]的研究表明ADV及TDF與臨床估算的腎小球濾過率(eGFR)降低相關,不建議應用于HBV-GN患者的治療。
恩替卡韋(ETV)及富馬酸丙酚替諾福韋酯(TAF)作為指南推薦的慢性腎臟病、腎功能不全或接受腎臟替代治療的一線抗HBV藥物,具有較高的腎臟安全性。其中ETV用于肌酐清除率<50 mL/min患者時需調整劑量,而TAF在eGFR≥15 mL·min-1·1.73 m-2的患者中均不需要調整劑量[36]。Notsumata等[37]的研究納入了38例由ETV轉換為TAF治療的HBV患者,治療后腎功能相關標志物如eGFR、尿肝臟型脂肪酸結合蛋白及血清成纖維細胞生長因子23均較轉換前明顯改善。同樣地,Ogawa等[38]的研究共納入313例應用ETV或NUC治療2年以上后轉換為TAF的HBV患者,腎功能相關標志物也均較轉換前顯著改善。由此可見,HBV-GN患者應用TAF治療具有更為顯著的獲益。
3.3 NUC聯合免疫抑制劑 免疫抑制劑在抑制免疫應答、減緩腎病進展方面具有不可替代的重要作用,目前臨床上多與抗病毒藥物聯合應用,不建議單用于HBV-GN的治療。一項納入317例HBV-GN患者的Meta分析[39]結果顯示,抗病毒藥物聯合激素治療與單用抗病毒藥物相比可顯著降低患者的尿蛋白水平,用藥過程中未觀察到肝腎功能的惡化。多項研究[40-41]比較了他克莫司聯合ETV與ETV單藥治療的效果,同樣觀察到了聯合治療組尿蛋白水平的顯著降低,并且聯合免疫治療未使HBV復制較前活躍。
4 結語
HBV-GN作為乙型肝炎常見合并癥之一,對乙型肝炎患者的預后具有重要影響。目前,HBV-GN的發病機制仍不明確,尚缺乏簡便特異的診斷方法及規范的治療方案,仍需更大規模、更高質量的研究進一步探尋,為臨床治療提供新的靶點及思路,做到早期診斷及治療,改善乙型肝炎患者的預后。
利益沖突聲明:所有作者均聲明不存在利益沖突。
作者貢獻聲明:李朝霞負責文獻檢索和撰寫;李楠負責文獻審核,修改文章;辛桂杰負責擬定寫作思路,終審定稿。
參考文獻:
[1]SCHWEITZER A, HORN J, MIKOLAJCZYK RT, et al. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013[J]. Lancet, 2015, 386(10003): 1546-1555. DOI: 10.1016/S0140-6736(15)61412-X.
[2]Polaris Observatory Collaborators. Global prevalence, treatment, and prevention of hepatitis B virus infection in 2016: a modelling study[J]. Lancet Gastroenterol Hepatol, 2018, 3(6): 383-403. DOI: 10.1016/S2468-1253(18)30056-6.
[3]HUANG Y, SHI KW, ZHU XJ, et al. Disease spectrum of 9310 cases of renal biopsy pathological diagnosis from a single center in China[J]. J Cent South Univ(Med Sci), 2022, 47(5): 546-554. DOI: 10.11817/j.issn.1672-7347.2022.210487.
黃瑤, 施克雯, 朱雪婧, 等. 中國單中心9310例腎活檢病理診斷疾病譜[J]. 中南大學學報(醫學版), 2022, 47(5): 546-554. DOI: 10.11817/j.issn.1672-7347.2022.210487.
[4]COMBES B, SHOREY J, BARRERA A, et al. Glomerulonephritis with deposition of Australia antigen-antibody complexes in glomerular basement membrane[J]. Lancet, 1971, 2(7718): 234-237. DOI: 10.1016/s0140-6736(71)92572-4.
[5]FERDEK PE, JAKUBOWSKA MA, GERASIMENKO JV, et al. Bile acids induce necrosis in pancreatic stellate cells dependent on calcium entry and sodium-driven bile uptake[J]. J Physiol, 2016, 594(21): 6147-6164. DOI: 10.1113/JP272774.
[6]DRING B, LTTEKE T, GEYER J, et al. The SLC10 carrier family: transport functions and molecular structure[J]. Curr Top Membr, 2012, 70: 105-168. DOI: 10.1016/B978-0-12-394316-3.00004-1.
[7]YAN H, ZHONG G, XU G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus[J]. Elife, 2012, 1: e00049. DOI: 10.7554/eLife.00049.
[8]WANG R, WU Y, ZHENG B, et al. Clinicopathological characteristics and prognosis of hepatitis B associated membranous nephropathy and idiopathic membranous nephropathy complicated with hepatitis B virus infection[J]. Sci Rep, 2021, 11(1): 18407. DOI: 10.1038/s41598-021-98010-y.
[9]LI D, GAO G, JIANG H, et al. Hepatitis B virus-associated glomerulonephritis in HBsAg serological-negative patients[J]. Eur J Gastroenterol Hepatol, 2015, 27(1): 65-69. DOI: 10.1097/MEG.0000000000000236.
[10]PRESCOTT NA, BRAM Y, SCHWARTZ RE, et al. Targeting hepatitis B virus covalently closed circular DNA and hepatitis B virus X protein: recent advances and new approaches[J]. ACS Infect Dis, 2019, 5(10): 1657-1667. DOI: 10.1021/acsinfecdis.9b00249.
[11]LIANG DY, SHA S, YI Q, et al. Hepatitis B X protein upregulates decoy receptor 3 expression via the PI3K/NF-κB pathway[J]. Cell Signal, 2019, 62: 109346. DOI: 10.1016/j.cellsig.2019.109346.
[12]HAN W, LUO M, HE M, et al. HBx gene transfection affects the cycle of primary renal tubular epithelial cells through regulating cyclin expression[J]. Mol Med Rep, 2018, 18(2): 1947-1954. DOI: 10.3892/mmr.2018.9197.
[13]LI M, HU L, ZHU F, et al. Hepatitis B virus X protein promotes renal epithelial-mesenchymal transition in human renal proximal tubule epithelial cells through the activation of NF-κB[J]. Int J Mol Med, 2016, 38(2): 513-520. DOI: 10.3892/ijmm.2016.2637.
[14]FENG Y, CUI Y, GAO JL, et al. Resveratrol attenuates neuronal autophagy and inflammatory injury by inhibiting the TLR4/NF-κB signaling pathway in experimental traumatic brain injury[J]. Int J Mol Med, 2016, 37(4): 921-930. DOI: 10.3892/ijmm.2016.2495.
[15]YANG YT, WANG X, ZHANG YY, et al. The histone demethylase LSD1 promotes renal inflammation by mediating TLR4 signaling in hepatitis B virus-associated glomerulonephritis[J]. Cell Death Dis, 2019, 10(4): 278. DOI: 10.1038/s41419-019-1514-4.
[16]ZHOU Y, ZHU N, WANG X, et al. The role of the toll-like receptor TLR4 in hepatitis B virus-associated glomerulonephritis[J]. Arch Virol, 2013, 158(2): 425-433. DOI: 10.1007/s00705-012-1508-3.
[17]LEE WM. Hepatitis B virus infection[J]. N Engl J Med, 1997, 337(24): 1733-1745. DOI: 10.1056/NEJM199712113372406.
[18]STARKE A, LINDENMEYER MT, SEGERER S, et al. Renal tubular PD-L1 (CD274) suppresses alloreactive human T-cell responses[J]. Kidney Int, 2010, 78(1): 38-47. DOI: 10.1038/ki.2010.97.
[19]LEE VW, QIN X, WANG Y, et al. The CD40-CD154 co-stimulation pathway mediates innate immune injury in adriamycin nephrosis[J]. Nephrol Dial Transplant, 2010, 25(3): 717-730. DOI: 10.1093/ndt/gfp569.
[20]WANG X, WANG L, ZHU N, et al. Hepatitis B virus X protein modulates renal tubular epithelial cell-induced T-cell and macrophage responses[J]. Immunol Cell Biol, 2016, 94(3): 266-273. DOI: 10.1038/icb.2015.85.
[21]WANG X, ZHOU Y, ZHU N, et al. The deposition of Notch1 in hepatitis B virus-associated nephropathy and its role in hepatitis B virus X protein-induced epithelial-mesenchymal transdifferentiation and immunity disorder in renal tubular epithelial cells[J]. J Viral Hepat, 2014, 21(10): 734-743. DOI: 10.1111/jvh.12244.
[22]ZHEN J, ZHANG L, PAN J, et al. AIM2 mediates inflammation-associated renal damage in hepatitis B virus-associated glomerulonephritis by regulating caspase-1, IL-1β, and IL-18[J]. Mediators Inflamm, 2014, 2014: 190860. DOI: 10.1155/2014/190860.
[23]BHIMMA R, HAMMOND MG, COOVADIA HM, et al. HLA class I and II in black children with hepatitis B virus-associated membranous nephropathy[J]. Kidney Int, 2002, 61(4): 1510-1515. DOI: 10.1046/j.1523-1755.2002.00287.x.
[24]PARK MH, SONG EY, AHN C, et al. Two subtypes of hepatitis B virus-associated glomerulonephritis are associated with different HLA-DR2 alleles in Koreans[J]. Tissue Antigens, 2003, 62(6): 505-511. DOI: 10.1046/j.1399-0039.2003.00141.x.
[25]HUI D, YAN X, WEI J, et al. Significance of mutations in hepatitis B virus X gene for the pathogenesis of HB-associated glomerulonephritis[J]. Acta Virol, 2014, 58(3): 278-281. DOI: 10.4149/av_2014_03_278.
[26]CHENG WR, SUN LJ, DONG HR, et al. Clinical and pathological characteristics of hepatitis B virus -associated glomerulonephritis in adults[J]. Int J Virol, 2021, 28(3): 231-235. DOl: 1073760/maj.sis 1673-4092.2021.03.013.
程文榮, 孫麗君, 董鴻瑞, 等. 成人乙型肝炎病毒相關性腎小球腎炎的臨床及病理特點分析[J]. 國際病毒學雜志, 2021, 28(3): 231-235. DOl: 1073760/maj.sis 1673-4092.2021.03.013.
[27]FRESQUET M, JOWITT TA, GUMMADOVA J, et al. Identification of a major epitope recognized by PLA2R autoantibodies in primary membranous nephropathy[J]. J Am Soc Nephrol, 2015, 26(2): 302-313. DOI: 10.1681/ASN.2014050502.
[28]XIE Q, LI Y, XUE J, et al. Renal phospholipase A2 receptor in hepatitis B virus-associated membranous nephropathy[J]. Am J Nephrol, 2015, 41(4-5): 345-353. DOI: 10.1159/000431331.
[29]DONG H, XU Y, XU T, et al. The role of HBx gene mutations in PLA 2R positive hepatitis-B-associated membranous nephropathy[J]. Biomed Environ Sci, 2020, 33(4): 269-272. DOI: 10.3967/bes2020.036.
[30]TU TQ, ZHENG XM, YANG Y, et al. Role of serum anti-phospholipase A2 receptor antibody level in diagnosis and prognosis assessment of hepatitis B virus-related membranous nephropathy[J]. J Chin Pract Diagn Ther, 2021, 35(1): 7-10. DOI: 10.13507/j.issn.1674-3474.2021.01.002.
涂天琪, 鄭旭敏, 楊悅, 等. 血清抗磷脂酶A2受體抗體水平在乙型肝炎病毒相關膜性腎病診斷及預后評估中的價值[J]. 中華實用診斷與治療雜志, 2021, 35(1): 7-10. DOI: 10.13507/j.issn.1674-3474.2021.01.002.
[31]RADHAKRISHNAN J, CATTRAN DC. The KDIGO practice guideline on glomerulonephritis: reading between the (guide)lines-application to the individual patient[J]. Kidney Int, 2012, 82(8): 840-856. DOI: 10.1038/ki.2012.280.
[32]SHAH HH, PATEL C, JHAVERI KD. Complete remission of hepatitis B virus-associated nephrotic syndrome from IgA nephropathy following peginterferon therapy[J]. Ren Fail, 2013, 35(2): 295-298. DOI: 10.3109/0886022X.2012.745785.
[33]LAI MW, CHANG MH. Updates in the management of hepatitis B in children[J]. Expert Rev Gastroenterol Hepatol, 2019, 13(11): 1065-1076. DOI: 10.1080/17474124.2019.1686975.
[34]FU B, JI Y, HU S, et al. Efficacy and safety of anti-viral therapy for Hepatitis B virus-associated glomerulonephritis: A meta-analysis[J]. PLoS One, 2020, 15(1): e0227532. DOI: 10.1371/journal.pone.0227532.
[35]UDOMPAP P, KIM D, AHMED A, et al. Longitudinal trends in renal function in chronic hepatitis B patients receiving oral antiviral treatment[J]. Aliment Pharmacol Ther, 2018, 48(11-12): 1282-1289. DOI: 10.1111/apt.15020.
[36]Chinese Society of Infectious Diseases, Chinese Medical Association, Chinese Society of Hepatology, Chinese Medical Association. Guidelines for the prevention and treatment of chronic hepatitis B (version 2019)[J]. J Clin Hepatol, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.
中華醫學會感染病學分會, 中華醫學會肝病學分會. 慢性乙型肝炎防治指南(2019年版)[J]. 臨床肝膽病雜志, 2019, 35(12): 2648-2669. DOI: 10.3969/j.issn.1001-5256.2019.12.007.
[37]NOTSUMATA K, NOMURA Y, TANAKA A, et al. Early changes in tubular dysfunction markers and phosphorus metabolism regulators as a result of switching from entecavir to tenofovir alafenamide fumarate nucleoside analog therapy for chronic hepatitis B patients[J]. Hepatol Res, 2020, 50(3): 402-404. DOI: 10.1111/hepr.13451.
[38]OGAWA E, NOMURA H, NAKAMUTA M, et al. Tenofovir alafenamide after switching from entecavir or nucleos(t)ide combination therapy for patients with chronic hepatitis B[J]. Liver Int, 2020, 40(7): 1578-1589. DOI: 10.1111/liv.14482.
[39]ZHENG XY, WEI RB, TANG L, et al. Meta-analysis of combined therapy for adult hepatitis B virus-associated glomerulonephritis[J]. World J Gastroenterol, 2012, 18(8): 821-832. DOI: 10.3748/wjg.v18.i8.821.
[40]WANG L, YE Z, LIANG H, et al. The combination of tacrolimus and entecavir improves the remission of HBV-associated glomerulonephritis without enhancing viral replication[J]. Am J Transl Res, 2016, 8(3): 1593-1600.
[41]XIE XF, XIE BY, ZHANG WH, et al. The efficacy and safety of tacrolimus and entecavir combination therapy in the treatment of hepatitis B virus-associated glomerulonephritis: a multi-center, placebo controlled, and single-blind randomized trial[J]. Ann Palliat Med, 2022, 11(5): 1762-1773. DOI: 10.21037/apm-22-328.
收稿日期:
2022-06-23;錄用日期:2022-08-08
本文編輯:王瑩

